Before the comparison of SN1 vs SN2 reactions, we briefly explain the nucleophilic substitution reaction. A nucleophilic substitution is a class of chemical reactions in which an electron-rich species (nucleophile) replaces a functional group within another electron deficient molecule (electrophile).
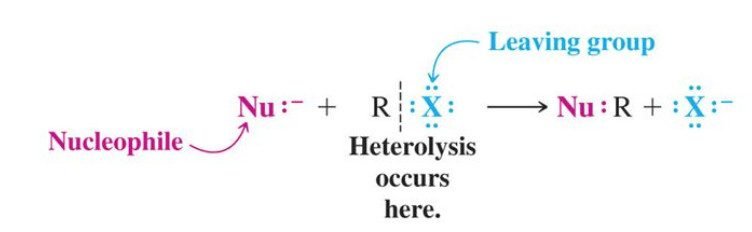
There are many types of nucleophilic substitution reactions:
- Nucleophilic substitution unimolecular
- Nucleophilic substitution bimolecular
- Nucleophilic substitution intramolecular
- Nucleophilic substitution aromatic
SN1 and SN2 | Nucleophilic Substitution Reaction
Click the SN1 and SN2 | Nucleophilic Substitution Reaction to know about the complete concepts of nucleophilic substitution reactions with the mechanism.
Comparison of SN1 vs SN2 Reactions
| SN1 Reaction | SN2 Reaction |
|---|---|
| SN1 stands for substitution nucleophilic unimolecular. | SN2 stands for substitution nucleophilic bimolecular. |
| The molecularity of the SN1 reaction is 1. | The molecularity of the SN2 reaction is 2. |
| R-X → R+ + X– (slow) R+ + Nu– →R-Nu (fast) | R-X + Nu– → X– (slow) |
| Rate ∝ [R-X] Rate = K [R-X] | Rate ∝ [R-X] [Nu] Rate = K [R-X] [Nu] |
| The coming nucleophile may attack from any side. | The coming nucleophile only attacks from the backside. |
| Tertiary alkyl halide generally gives an SN1 reaction. | Primary alkyl halides generally give the SN2 reaction. |
| The product is a racemic mixture i.e. 50% inversion and 50% retention of configuration. | The product is with 100% inversion of configuration. |
| The order of the reaction is 1. | The order of the reaction is 2. |
| It is a two-step mechanism. | It is a single-step mechanism. |
| It is favored in the polar solvent. | It is favored in the nonpolar solvent. |
Some Important Points About SN1 Reaction
- SN1 reaction does not depend upon the nucleophile.
- SN1 reaction depends upon the nature of alkyl halide. The nature of alkyl halide means the formation of the most stable carbocation. The order of stability of carbocation is:
Tertiary > Secondary > Primary
- If we take a very low concentration of nucleophile, no effect on the reaction.
- The formation of the carbocation is the rate-determining step.
- The rearrangement of the carbocation is take place (hydride shift, methyl shift to form the most stable carbocation), this will not affect the rate of reaction.
- The solvent used in the SN1 reaction is polar protic solvent.
R-X
The bond between R-X is break due to polar nature of solvent, and this property is called dielectric property. Water is a good dielectric property and decrease the electrostatic force between R-X. The dielectric constant of water is 80. That’s why the solvent is polar in nature. Now the question is that why solvent is protic in nature?
Protic mean H+. When the leaving group leave the substrate after the attack of nucleophile, the water molecule surrounds the leaving group due to its protic property. Some of the molecules of water surrounds the X– or leaving group Nu– like a cage and as we know water is a solvent (in excess amount) so the remaining water molecule react with carbocation. That’s why the carbocation not recombined with the living group Nu– but reacts with water. So, the water attack on carbocation and reaction proceeds forward.
- H2O, C2H5OH,CH3OH are most comon polar protic solvent. CH3COOH also act as polar protic solvent.
- SN1 is exothermic in nature. As we know that the exothermic reactions favour at low temperature and at low temperature reaction moves forward (Le-chateliear principle) so we can say that the SN1 reaction prefer at low temperature.
- The SN1 reaction gives the racemic mixture when alkyl halide is chiral.
Some Important Points About SN2 Reaction
- In SN2 reaction, the nucleophile is attacked on alkyl halide opposite to the halide. (back side)
- No intermediate formation, In SN2 reaction, transition state is formed.
- In SN2 reaction, only inversion of configuration because nucleophile attacked on alkyl halide from back side.
- In rds, two molecules are involved are called bimolecular nucleophlic substitution.
- There is no formation of carbocation.
- Transtion state is firmed and it cannot be isolated from reaction.
- Thw more stable the transition state, the more will be the SN2 reaction.
- SN2 is exothermic in nature. As we know that the exothermic reactions favour at low temperature and at low temperature reaction moves forward (Le-chateliear principle) sothe SN1 and SN2 reactions both prefer at low temperature.
Hope so the above discussion will helpful to understand the comparison of SN1 vs SN2 reactions.


Leave a Reply