Slater’s Rule is basically used to calculate the effective nuclear charge in an atom. To understand the effective nuclear charge, it is important to understand the shielding effect or screening effect. The shielding effect or screening effect occurs when there is the presence of intervening electrons which depresses the force of attraction towards the outermost electron present in the outermost shell.
Shielding or Screening Effect:
Shielding effect is defined as:
- A decrease in the force of attraction between the nucleus and valance electrons due to incoming and intervening electrons is called the screening effect.
- Basically, the shielding effect is used to describe the balance between the force of repulsion between inner electrons and the attractive forces between the protons and electrons.
- So, the force between the protons and electrons is called the effective nuclear charge. So, we can calculate the effective nuclear charge very easily but we should calculate the screening constant before calculating the Zeff.
- It is also used to explain the removal of electrons from the outermost shell, atomic size & radii of the atom as well as all the periodic properties like ionization energy, electron affinity and, electronegativity.
- By the following relation “Fnet = Fattraction – Frepulsion“ it is noticed that if there are several electrons in an atom then there are higher repulsive forces between them. Higher repulsive forces mean weakness of attractive forces so there is a decrease in effective nuclear charge.
Effective nuclear charge & Shielding effect:
Due to increased repulsive forces in inter electron of an atom, there is a reduction in the nuclear charge because of the shielding effect or screening effect. A reduced nuclear charge is called an effective nuclear charge. Effective nuclear charge is denoted by Zeff. Basically effective nuclear charge is the net charge which is experienced at the outermost electron due to intervening electrons.
Ineffective nuclear charge, the term “Effective” refers to the shielding effect of electrons which disallows higher energy orbits electrons to experience full nuclear charge. The shielding effect of inter electrons is called core charge. Effective nuclear charge is measured by the following formula:
Zeff = Z – σ
In this formula, it is easy to calculate the effective nuclear charge. Z is the atomic number of an atom and σ is the shielding or screening constant “Screening constant is the measure of repulsion between the inter electrons“. Now we are going to discuss a relation between the effective nuclear charge, σ Shielding constant, and interelectronic repulsion.
Inter electronic repulsion ∝ Screening constant ∝ 1/Zeff
The above relation discussed is showing that an increase in interelectronic repulsion causes an increase in the screening constant and a decrease in the effective nuclear charge. This is because when the atomic number increases the number of electrons and protons increase so inner electrons repulsive forces dominate.
Trends of Shielding effect:
Along with the group, there is a decrease in the shielding effect because of increased atomic size. The increase in atomic size is due to the addition of the number of shells. So, the atomic size gets large and there is less interelectronic repulsion, so there is less shielding effect and greater effective nuclear charge. This decrease shielding effect may cause a decrease in the ionization energy values and an election is ionized with less force. Similarly, electronegativity and electron affinity decreases.
Along with the period, there is an increase in the shielding effect because of the decrease in atomic size. The decrease in the atomic size is due to the addition of more and more electrons. This addition may cause an increase in the electrostatic force of attraction. So, by an increase in no. of electrons inner electrons repulsive forces dominate, and the effective nuclear charge becomes lesser. So, there is an increase in ionization energy, electron affinity, and electronegativity.
Slater’s Rule:
Slater’s Rule is used to find the effective nuclear charge by knowing the contribution to the screening constant. There are some of the most important rules that are used to calculate the effective nuclear charge:
- First of all, an electronic configuration is written such as 1s2, 2s2, 2p6, 3s2, 3p6……… (in case of s and p orbitals).
- But in the case of d orbital electronic configuration is written as 1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s2….. but in this case s orbital is filled first and then d orbital is filled later.
- Because electrons feel stability when they are filled in the lower energy orbit and then higher energy orbit. Since electrons must fill in the lower energy state first.
- For one electron (1e–) the constribution of screening constant is 0.3. i.e. He = 1s2
We will take 0.3 for only one electron because there is one electron present for that we can calculate Effective nuclear charge. We cannot consider that electron which is involved in inter repulsive forces. because one electron never repels and attracts itself.
So, σ = 0.3 * 1 (This 1 is representing the number of electrons for which we are calculating effective nuclear charge)
σ = 0.3 (this value is for screening constant)
Zeff = Z – σ
Zeff = 2 – 0.3
Zeff = 1.70
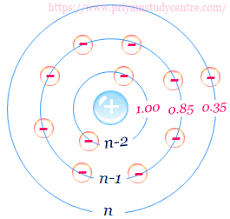
- Screening effect of ns and np (outermost orbit) electrons is 0.35. For example: Li, Be, C, N, O, F etc
- Screening effect of (n-1) s, p, d (Penultimate orbits) eletron is 0.85. For example: Li, Be, C, N, O, F etc
- Screening effect of (n-2) electrons and below in s, p, d, f is 1.0.
Calculation of Effective nuclear charge & Screening constant:
We will calculate the effective nuclear Charge and screening constant of the 2nd period:
1. Lithium (Li), Z= 3, 1s2, 2s1.
σ = 0.85 * 2 (2 is the number of electrons. We take 2 electrons because 3rd is neither itself repel nor attract).
σ = 1.70
Zeff = Z – σ
Zeff = 3 – 1.70
Zeff = 1.30
2. Berillium (Be), Z= 4, 1s2, 2s2.
σ = 0.35 * 1 + 0.85 * 2 (In this case n and n-1 both are included. So, ns = 0.35 & n-1 = 0.85)
σ = 2.05
Zeff = Z – σ
Zeff = 4 – 2.05
Zeff = 1.95
3. Boron (B), Z= 5, 1s2, 2s2, 2p1.
σ = 0.35 * 2 + 0.85 * 2
σ = 2.40
Zeff = Z – σ
Zeff = 5 – 2.40
Zeff = 2.60
4. Carbon (C), Z= 6, 1s2, 2s2, 2p2.
σ = 0.35 * 3 + 0.85 * 2
σ = 2.75
Zeff = Z- σ
Zeff = 6 – 2.75
Zeff = 3.25
5. Nitrogen (N), Z= 7, 1s2, 2s2, 2p3.
σ = 0.35 * 4 + 0.85 * 2
σ = 3.10
Zeff = Z – σ
Zeff = 7 – 3.10
Zeff = 3.90
| ELEMENT | ATOMIC NUMBER | Calculation for nth shell (0.35 * n) | Calculation for n-1 shell (0.85 * 2) | Screening constant (σ) | Effective Nuclear charge (Zeff) |
| Lithium | 3 | – | 1.70 | 1.70 | 1.30 |
| Beryllium | 4 | 0.35 * 1 = 0.35 | 1.70 | 2.05 | 1.95 |
| Boron | 5 | 0.35 * 2 = 0.70 | 1.70 | 2.40 | 2.60 |
| Carbon | 6 | 0.35 * 3 = 1.05 | 1.70 | 2.75 | 3.25 |
| Nitrogen | 7 | 0.35 * 4 = 1.40 | 1.70 | 3.10 | 3.90 |
| Oxygen | 8 | 0.35 * 5 = 1.75 | 1.70 | 3.45 | 4.55 |
| Flourine | 9 | 0.35 * 6 = 2.10 | 1.70 | 3.80 | 5.20 |
It is noticed that the following results are drawn from the above table:
- When we move from left to right to left in a period, calculations for the nth shell increases because several electrons are added.
- And calculation for the n-1 shell remains the same because electrons are added in the outermost shell, not in the inner shell.
- So, on this behalf screening constant increases showing the contribution of intervening electrons in the shielding effect.
- Hence, Effective nuclear charge also increases gradually.
Calculation of Zeff in the d-block elements by slater’s rule:
d-block elements are the elements that require d orbital for their completion and to attain stability. Following are the d-block elements:
1. Scandium (Sc), Z= 21, 1s2, 2s2, 2p6, 3s2, 3p6, 3d1, 4s2
The point to notice is that electrons should always fill in the lower energy orbit because atoms become stable if electrons fill first in the lower energy state. According to this relation, 4s > 3d electrons fill first in 4s.
σ = 0.35 * 1 + 0.85 * 9 + 1 * 10
σ = 18
Zeff = Z – σ
Zeff = 21 – 18
Zeff = 3
2. Titanium (22), 1s2, 2s2, 2p6, 3s2, 3p6, 3d2, 4s2
σ = 0.35 * 1 + 0.85 * 10 + 1 * 10
σ = 18.85
Zeff = Z – σ
Zeff = 22 – 18.85
Zeff = 3.15
Exceptional Electronic configuration:
There are some cases in which there exist exceptional electronic configurations. These exceptional configurations occur because an atom such as Chromium or copper wants to attain stability. This is because of Energy exchange.
In the case of Chromium:
Z = 24, 1s2, 2s2, 2p6, 3s2, 3p6, 3d4, 4s2 (NORMAL CONFIGURATION)
1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1 (EXCEPTIONAL CONFIGURATION)
In case of Chromium and copper there is energy exchange method which follow symmetrical distribution of electronic cloud and so that half filled orbital is more stable then that of partly filled orbitals: HALF FILLED >> PARTLY FILLED.
Chromium (Cr), Z = 24, 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1 (EXCEPTIONAL CONFIGURATION)
σ = 0.35 * 0 + 0.85 * 13 + 10 * 1
σ = 21.05
Zeff = Z – σ
Zeff = 24 – 21.05
Zeff = 2.95
Copper (Cu), Z = 29, 1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s1 (EXCEPTIONAL CONFIGURATION)
This type of configuration occurs due to the symmetrical distribution of electronic cloud so that 3d10 >> 3d9
So, its effective nuclear charge is 3.70. COMPLETELY FILLED >> HALF FILLED >> PARTIALLY FILLED
Periodic variation Of Zeff:
- Zeff increases from left to right in a period.
- That’s why atomic size decreases.
- Atomic number (Z) is increased by 1 but the screening effect increases by 0.85 so, Zeff is 0.15 because electrons enter in (n-1) orbit that has a value of s = 0.85.
- In transition series, Zeff increases in very less amount by 0.15 from left to right, and hence atomic size remain constant.
- From top to bottom Zeff remains constant.
CONCLUSION:
So, in this article, we have discussed in detail, the screening effect, Slater’s rule, and periodic variations of Zeff. In a periodic variation of Zeff, the trend is opposite to the periods. If there is an increase in Zeff in periods then there will be a decrease in the Zeff trend.


Leave a Reply