
Finding the concept of oxymercuration as a reaction is important. Understanding what is Markonikove’s rule is important to understanding oxymercuration reaction. Oxymercuration reaction is also called oxymercuration-demurcuration reaction. Markonikove’s rule predicts the regioselectivity of electrophilic addition reaction. These reactions are given by unsaturated hydrocarbons, i.e. alkenes. The reaction in which the reagent is attacked by an electrophile (electron lover) is called an electrophilic addition reaction.
Markonikove’s Rule states that:
“If any electrophile such as HX attacks on a reagent, H+ will attack to that side of unsymmetrical alkene which has greater number of hydrogen atom, but the halide group attaches itself to carbon atom having greater number of alkyl substituents”
CH3 – CH = CH2 + HBr → CH3 CH-Br CH3
What is oxymercuration reaction??
Oxymercuration reactions are the electrophilic addition organic reactions by which an unsaturated alkene is transferred into alcohol (having no charge on alcohol). An unsaturated alkene get reacts with the mercuric acetate Hg(OAc)2 in an aqueous medium to produce an acetoxymercury group (HgOAc) and a hydroxyl group (OH).
Yielding carbocation rearrangement is a spontaneous process by which a stable carbocation is formed. We cannot get it stage of carbocation formation because this reaction is so fast under mild conditions and 90% of the product is yielded.
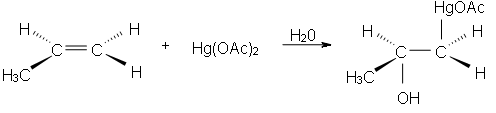
Now, this intermediate formed is not our desired product. So, take our desired product we react it with the Sodium borohydrate NaBH4, NaOH & H2O to form desired products (alcohol). The addition of the attacking nucleophile will cause to obtain the product on the basis of Markonikove’s Regioselectivity.
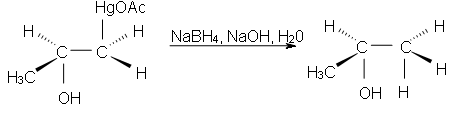
How do oxymercuration reactions occur?? Mechanism:
It is the electrophilic addition reaction that occurs in three steps. This whole process is sometimes called deoxymercuration. The step-wise illustration of oxymercuration reaction is as under:
a) Step 1:
In the first step, dissociation of mercuric acetate occurs. Mercuric acetate breaks down into two acetoxymercuric groups. When the reagent reacts with the acetoxymercuric group the nucleophile attacks the Mercury iron from which the Acetoxy group is ejected. The carbon on the double bond is attacked by the lone pairs of the Mercury forming the mucuronium ion, having a positive charge on the Mercury ion.
This occurred due to the donation of electrons in the highest molecular orbital to the empty 6s orbital and also electrons donated to the lowest unoccupied orbitals by the eg (dz2, dx2-y2) orbitals of Mercury. So, in this step acetoxy mercuronium ion forms a cycle on both carbons of alkenes.
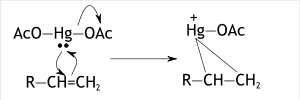
b) Step 2:
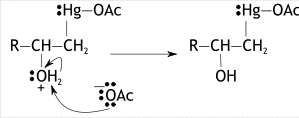
Now, in the second step, water will act as a nucleophile. The attack of nucleophile occurs at more substituted carbon involves in bond cleavage and electrons forming bonds destruct to mercury ion and neutralizes it. Now, oxygen present in water (aqueous medium) will now bear a positive charge.

c) Step 3:
In the third step, the acetoxy ion ejected in the first step attacks the hydrogen ion present in water producing a by-product HOAc. This attack on nucleophile causes the bond to break between oxygen and hydrogen which neutralizes a charge on oxygen & in last our required product alcohol is formed.
Stereochemistry of oxymercuration reaction:
Understanding the concept of the stereochemistry of oxymercuration reaction is necessary. In the following reaction when H2O reacts it acts as a nucleophile. It follows markonikove’s regioselectivity and attack on that carbon which is more substituted. Attacking of nucleophiles causes deposition of a hydroxyl group. Hence, murcuronium ion is formed therefore positive charge on mercury is resided by the most substituted carbon form temporary tertiary carbocation which is positive so acts as an electrophile.
Nucleophile attacks on electrophile’s most substituted carbon because it wants to retain more positive character to become a more stable compound than less substituted carbon.
In the second step, when the cyclic structure is formed, it has a higher steric hindrance. From the front side, stereochemistry is blocked. So, nucleophile attacks from the backside. There will be no free rotation & nucleophile attacks from the opposite sides. So, it forms trans compounds to each other. Therefore, oxymercuration-demurcuration is an anti-addition reaction. Anti-addition refers to the addition of nucleophiles from the opposite side where there is no blockage of stereochemistry.


Leave a Reply