Molecularity is the number of molecules or atoms of reactant that takes part in an elementary (single step) chemical reaction. We can say how many molecules of reactant collide effectively to give a product. According to collision theory, molecules effectively collide and give a product. The word effectively means at a proper angle and at proper energy or particular energy.
What is Molecularity in Chemistry?
The number of molecules or atoms of reactant that must collide simultaneously and effectively to give the product in the elementary reaction is called molecularity. The concept of molecularity is only for elementary reactions. The molecularity is not defined for complex (multi-step) reactions.
What is Unimolecular Reaction with Example?
Unimolecular mean the rate determining step of these reactions depends upon only one molecule. For example:
H2O2 (l) → H2O (l) + 1/2 O2 (g)
H2O2 in liquid phase decomposes producing water and half of the oxygen gas. In this case, only one molecule of hydrogen peroxide can produce water and oxygen. That’s why it is a unimolecular reaction. The SN1 reaction is unimolecular reactions because in SN1 reactions, one molecule is involve in rate determining step. SN1 reactions are also known as first order reaction.
What is Bimolecular Reaction with Example?
Bimolecular mean the rate determining step of these reactions depends upon two molecules. When the reaction involves collision between two species it will be said as bimolecular reaction. For example:
2HI(g) → H2 (g)+ I2 (g)
Two molecules of hydrogen iodide produces hydrogen gas and iodine gas, so here two molecules are used that’s why it is bimolecular reaction. The SN2 reaction is bimolecular reactions because in SN2 reactions, two molecules are involve in the rate determining step. SN2 reactions are also known as second order reaction.
What is Termolecular Reaction with Example?
If there are three molecules or species are reacting to produce the product and it will be called as termolecular reactions. For example:
2NO(g) + O2 (g) → 2NO2 (g)
The two molecules of nitrous oxide and one molecule of oxygen can combine to produce nitrogen dioxide. This is termoleclar reaction.
What is Elementary Reaction with Example?
It is the reaction that takes place in a single step and cannot be broken down into simpler chemical reactions. Let us a general elementary reaction:
A + B → C
The rate of elementary reaction is:
rate = K [A]1[B]1 (experimentaly)
- There is no intermediate product in elementary reactions.
- Transition state exist.
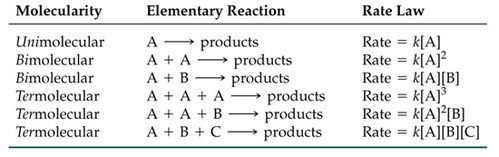
Molecularity of an Elementary Reactions
It is the number of reactant molecules taking part in the reaction.
Find the molecularity and order of the following reactions:
A + B → C (elementary)
Rate = K [A]1 [B]1 (experimentaly)
- Molecularity = 2
- Order = 1+1 = 2
The molecularity and order of reaction of elementary reaction is same.
NH4NO2 → N2 + 2H2O (elementary)
Rate = K [NH4NO2]1 (experimentaly)
- Molecularity = 1 (unimolecular reaction)
- Order = 1
2HI → H2 + I2 (elementary)
Rate = K [HI]2 (experimentaly)
- Molecularity = 2 (bimolecular reaction)
- Order = 2
2NO + O2 → 2NO2 (elementary)
Rate = K [NO]2[O2]1 (experimentaly)
- Molecularity = 3 (termolecular reaction)
- Order = 2+1 = 3
CH3COOC2H5 + H2O → CH3COOH + C2H5OH (elementary)
Rate = K [CH3COOC2H5]1[H2O]1 (experimentaly)
But in this reaction water act as solvent because it is freely available. So, [H2O] act as constant. Both K and [H2O] is constant.
K and [H2O] = K‘
Rate = K‘ [CH3COOC2H5]1
- Molecularity = 2 (bimolecular reaction)
- Order = 1
The reaction in which molecularity and order of reaction are not same is called Pseudo order reaction. This abover particular recation is called Pseudo first order reaction.
C12H22O11 + H2O → C6H12O6 + C6H12O6 (elementary)
Rate = K [C12H22O11]1[H2O]1 (experimentaly)
Similarly in this reaction, water act as solvent. So, [H2O] act as constant. Both K and [H2O] is constant.
K and [H2O] = K‘
Rate = K‘ [C12H22O11]1
- Molecularity = 2 (bimolecular reaction)
- Order = 1
It is pseudo first order reaction.
Note:
In all elementary reactions except pseudo order reactions, order is equal to molecularity.
Complex Recations:
- Complex reactions that occurs in several steps, where each step is elementary.
- Molecularity of complex reaction is not defined.
- Molecularity of each step is defined.
- Rate is given by slowest step of complex reactions.
- There will be intermediates in complex reactions.
- Intermediate should not appears in rate law of reaction.
NO2 + CO → NO + CO2 (complex)
rate = K [NO]2 (experimentaly)
The doesn’t depends upon CO.
- Order = 2
- Molecularity = not defined for complex reactions
CH3CHO → CH4 + CO (complex)
rate = K [CH3CHO]3/2 (experimentaly)
- Order = 3/2
- Molecularity = not defined for complex reactions
Molecularity is not defined for complex reactions. Molecularity is lawys less than or equal to 3. It is because the probability of simultaneous and effective collision of three molecules is very low. Hence, intermoleclar or higher molecuarity reactions are rarely observed.
Difference between Elementary Recation and Complex Recation
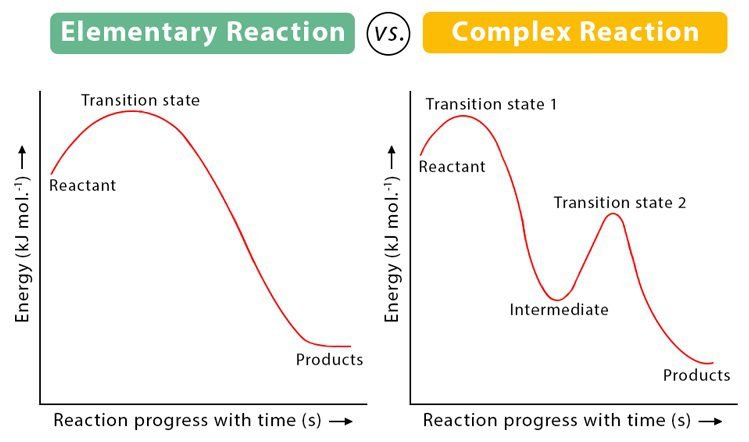
What is the basic difference between order and molecularity?
| Order of Reaction | Molecularity |
|---|---|
| Experimentaly determined | Theroratically determined |
| Defined for elementary as well as complex reactions | Defined only for elementary reactions |
| Order can be negative, positive, zero or fractional. | Molecularity is always positive. It can never be zero. negative or fractional. |
| Order can be greater than 3. | Molecularity can never greater than 3. |


Leave a Reply