
Mole fraction may be defined as the ratio of the number of moles of one component to the total number of moles of all the components (solute and solvent) present in a solution. It is donated by the letter “X” and the sum of all mole fractions in a solution is always equal to unity.
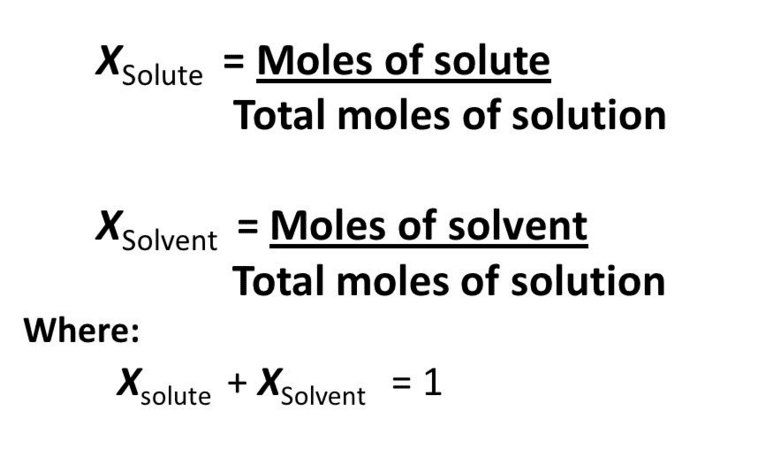
Mole fraction does not depend upon temperature and can be extended to solutions having more than two components.
How do you Calculate the Mole Fraction?
First we consider the general example to understand how we calculate the mole fraction of different components present in a solution.
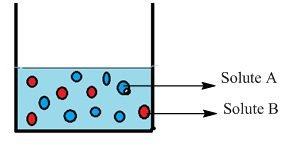
Let us a container having two solutes, solute “A” and solute “B”. The question is here, how do you calculate the mole fraction of both solutes “A” and “B”. The mole fraction of “A” is calculated by the number of moles of solute “A” divided by the number of moles of solute “A” and the number of moles of solute “B”.
XA = nA / nA + nB
Similarly, to calculate the mole fraction of solute B
XB = nB / nA + nB
A solution containing 40g sodium hydroxide and 54g water. Find the mole fraction of sodum hydroxide and water?
First we calculate the number of moles of NaOH:
nNaOH = mass of NaOH / Molar mass
nNaOH = 40 / 23 + 16 + 1
nNaOH = 40 / 40
nNaOH = 1
The number of moles of H2O: (can you burn water?)
nH2O= mass of H2O / Molar mass
nH2O = 54 / 2(1) + 16
nH2O = 54 / 18
nH2O = 3
Mole Fraction of NaOH:
XNaOH = nNaOH / nNaOH + nH2O
XNaOH = 1 / 1 + 3
XNaOH = 1/4
Mole Fraction of H2O:
XH2O = nH2O / nNaOH + nH2O
XH2O = 3 / 1 + 3
XH2O = 3/4
Is the sum of mole fraction of NaOH and H2O is equal to unity?
XNaOH = 1/4 = 0.25
XH2O = 3/4 = 0.75
XNaOH + XH2O = 1
0.25 + 0.57 = 1
1 = 1
Hence proved that the sum of all mole fraction is always unity.
A solution has 46% (w/w) ethyl alcohol in water. Find the mole fraction of ethyl alcohal?
46% (w/w) ethyl alcohol means 46g of ethyl alcohol in 100g of water. It means that in 100g of solution, 46g of ethanol and 54g of water is present.
nC2H5OH = mass of C2H5OH / molar mass
nC2H5OH = 46 / 46
nC2H5OH = 1
nH2O = mass of H2O / molar mass
nH2O = 54 / 18
nH2O = 3
Now we calculate the mole fraction of ethyl alcohol,
XC2H5OH = nC2H5OH / nH2O + nC2H5OH
XC2H5OH = 1 / 3 + 1
XC2H5OH = 1 / 4
After understand the concept of mole fraction and how to calculate it, now we discussed some concentration terms.
Some Concentration Terms
1. Wt by Wt %
A solution of 5% NaCl in water (wt by wt %), It means that 5g of NaCl is present in 100g of solution.
Formula:
wt by wt % = mass of solute / mass of solution × 100
10g of sucrose is disolve in 100g of water. Find wt by wt %?
10 g of sucrose (solute)
100g of water (solvent)
Solution = 10 + 100 = 110g
wt by wt % = mass of solute / mass of solution × 100
wt by wt % = 10 / 110 × 100
wt by wt % = 9.09 %
10g of NaCl is present in 100ml of solution. Find wt by wt %? If density of solution is 1.2 g/mol.
Density of solution = 1.2 g/mol
ρ = m/v
m = ρ × v
m = 1.2 g/mol × 100ml
m = 120g
wt by wt % = mass of solute / mass of solution × 100
wt by wt % = 10/120 × 100
wt by wt % = 8.33%
2. V by V %
A HCl aqeouse solution is 7% (V by V %). It means that 7ml HCl is present in 100ml of solution.
Formula
V by V % = volume of solute / volume of solution × 100
3. Wt by V %
A sugar solution 3% (wt by V %). It means that 3g of sugar (solute) is present in 100ml solution.
Formula
wt by V% = mass of solute / volume of solution × 100
Note:
- If wt by wt % then, 3g of sugar in 100g of solution
- If V by V % then, 3ml of sugar in 100ml of solution
- If wt by V % then, 3g of sugar in 100ml of solution
A sugar solution is 10% (w/v). Find (w/w%), if density of solution is 1.25 g/mol.
w/w % = ?
wt by wt % = mass of solute / mass of solution × 100
Given,
10% sugar solution (w/v %) means 10g of sugar in 100ml of solution.
mass of solute = 10g
mass of solution = ?
From density, ρ = m/v
m = ρ × v
m = 1.25 g/ml × 100ml
m = 125g
wt by wt % = mass of solute / mass of solution × 100
wt by wt % = 10g / 125g × 100
wt by wt % = 8%
A sugar solution is 10% (w/w %). Find (w/v %), if density of solution is 1.2 g/ml.
w/v % = ?
wt by v % = mass of solute / volume of solution × 100
Given,
10% sugar solution (w/w %) means 10g of sugar in 100g of solution.
mass of solute = 10g
volume of solution = ?
From density, ρ = m/v
V = m/ρ
V = 100g / 1.2 g/ml
V = 0.98 ml
wt by v % = mass of solute / volume of solution × 100
wt by v % = 10g / 0.98ml × 100
wt by wt % = 12%
A solution of HNO3 is 5% w/w. Find the mass of HNO3 present in 100ml of solution. (density of solution = 1.4 g/ml)
mass of HNO3 = ?
Solution of HNO3 IN w/w is 5%
From density, ρ = m/v
m = ρ × v
m = 1.4 × 100
m = 140g
wt by wt % = mass of solute / mass of solution × 100
5 = mass of HNO3 / 140 × 100
mass of HNO3 = 7g



Leave a Reply