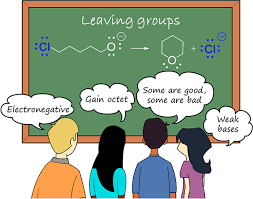
What is Leaving Group???
This blog will be based on the Leaving group and leaving group trends. To understand the concept of a leaving group, we shall understand the basic difference between a strong as well as a weak base. Basically, a lewis weak base will act as a nucleophile as well as leaving group.
G.N Lewis proposed a basic and clear difference between acid and base. According to him,
A species that has the ability to donate a pair of electrons is a strong base and has less electronegativity.
A weak Lewis base will possess some of the properties:
- A weak lewis base will have high electronegativity. High electronegativity means it does not attract the shared pair of electrons. As a result, it becomes a weak base. So, leaving groups are weak bases.
- A weak base or leaving group acts as a nucleophile.
Why strong base never acts as Leaving group??
A strong base will never act as a good leaving group because it is highly electronegative. A strong base allows the donation of lone pairs/electrons. So, the donation of an electron from a base depends upon three factors:
- Electronegativity: A strong base has less electronegativity. So, it cannot attract the shared pair of electrons more strongly due to less electronegativity. Hence, it loses the lone pair.
CH3–> NH2– > HO– > F
- Size of atom: An increase in the size of the atom causes a decrease in the strength of the H-X bond, and a lower electron density causes it to make a weak base. But the decrease in size causes an increase in the strength of the H-X bond, and higher the electron density causes it to make a strong base.
F– > Cl– > Br– > I–
- Resonance: Resonance is basically the delocalization of electrons. According to the concept of organic chemistry, a compound containing delocalization of electrons is considered to be a very stable compound so did not act as a weak base.
What is a good leaving group???
A good leaving group can be recognized as being a stable conjugate base of a strong acid. For example, water is a good leaving group as its conjugate base hydronium ion H3O+ is very stable because it can remove a proton very easily. A good leaving group can leave a compound very easily just like in nucleophilic substitution reactions.
In the nucleophilic substitution reaction, halogen is the best leaving group. Determining good leaving groups is important. That leaving groups are very good when departs take an electron pair from the compound. If accommodation of electrons makes a group more stable then it is a more good leaving group.
Leaving the group may also depend upon the basicity of a base. Many people confused the basicity with the basic character. Basicity and basic character are two opposite terms. Basicity refers to the ability of a base to accept a proton but basic character refers to the ability of an atom to act as a base. So, WEAKER THE BASICITY OF the GROUP, GOOD WILL BE THE LEAVING GROUP.
Is Cl or Br a good leaving the group???
Halogens are considered to be a good leaving group but these can also be determined by their difference in electronegativity. If we observe the order of good leaving group after iodine, bromine is considered to be a very good leaving group than chlorine. It is because chlorine has more electronegativity than bromine. SO, as we prove earlier chlorine is more strong base than bromine. But a weak base is a good leaving group. Therefore, Bromine is a good leaving group than chlorine.
General leaving group Trends:
Trends belong to the situation in which many variations occur in the periodic table in the periodic table. In this area, we will cover what factors are responsible for the variations of the good and bad leaving groups.
Variation in electronegativity:
From left to right in a period, atomic size and radius decrease. By decrease in size, ionization energy, electronegativity, and electron affinity increase due to an increase in the nuclear charge toward the outermost and also shielding effect decrease. All the parameters are followed.
An increase in electronegativity cause to increase in the attraction of shared pair of electron. So, basicity decreases, and the ability to leave the group to leave increases. So, as we discussed earlier weak base is a good leaving group and has more electronegativity.
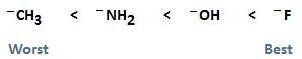
Variation in Atomic size:
In the periodic table, variations in atomic size may occur. And these variations lead to the evaluation of a good leaving group. An increase in atomic size is responsible for the decrease in nuclear charge and the decrease in electronegativity. An increase in size causes a decrease in basicity and as a result, increases the ability to leave the group. So, a larger size molecule may result in the evaluation of a good leaving group.
Resonating structures:
Resonance is basically the delocalization of electrons. Resonance in a compound makes it a weak base, and a base is a good leaving group.


Leave a Reply