Here is a complete overview of the bond order of H2. Bond order from the number of electrons in bonding and antibonding molecular orbits:
Before knowing the Bond order of H2 we should know about the molecular orbitals. When all the electrons occupying bonding molecular orbitals (Nb) and antibonding molecular orbitals (N*) are counted separately, the following cases arise:
- If the number of electrons in BMO’s is greater than that of those in ABMO’s, the molecule would be stable.
- If the number of electrons in BMO’s is equal to the number of electrons in ABMO’s, then the molecule is unstable and does not exist.
Bond order:
Bond order is the number of covalent bonds present in a molecule.
Bond order = 1/2 (Number of electrons in BMO – Number of electrons in ABMO)
Information conveyed by bond order:
- The bond order gives the nature of the bond, whether it is a single, double or triple.
- When the bond order comes out to be zero, there is no possibility of bond formation.
- As the bond order increase, bond length decrease.
- Bond dissociation energy is directly proportional to the bond order for diatomic molecules.
Magnetic property, Paramagnetic or Diamagnetic:
If the electric field is present, then a magnetic field is also present or vice versa. Both the electric field and magnetic field are opposite in direction. If the magnetic field of the coming atom does not attract or repeal an electron’s magnetic field, then diamagnetic. If the electron’s magnetic field is upward and the coming atom magnetic field is downward paramagnetic shows attraction. If the electron’s magnetic field and upcoming atom are upward or downward (in the same direction), then paramagnetic show repulsion.
- If the electron is paired, then Diamagnetic.
- If the electron is unpaired, then paramagnetic.
Molecular energy level diagram for hydrogen molecule (H2 ):

The overlap of 1s atomic orbitals forms H2. Its shell has two molecular orbitals. one is bonding (σ1s), and the other is antibonding (σ*1s). The H2 molecule has two electrons, which can be accommodated in the σ1s molecular orbital. Following the Pauli exclusion principle, this electron must have opposite spins.
Bond order of H2 :
As discussed above, Bond order is the number of covalent bonds present in a molecule.
For H2 , Bond order = 1/2 (2 – 0)=1
The bond order of H2 shows that hydrogen has only one bond. The BMO contains 2 electrons with opposite spins, and ABMO is empty. If the number of electrons in bonding orbitals is greater than the antibonding orbital, the molecule is stable. Thus, H2 is a stable molecule. There is no unpaired electron in the MO, so H2 is diamagnetic. Bond dissociation energy in H2 is 431.4 KJ mol-1, and bond length is 74 pm.
Molecular energy level diagram H2+:
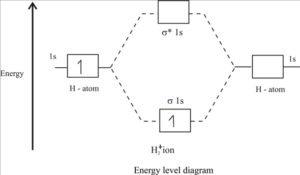
Bond order of H2+ :
H2+ means that the molecule of hydrogen loses its one electron. The H2+ molecule ion will have the electronic configuration [ (σ1s)1 ].
For H2+ , bond order = 1/2 (1 – 0)=1/2 σ bond.
Since the bond order in H2+ is only one-half of a standard covalent bond, it will have low bond dissociation energy and large bond length. There is an unpaired electron in the MO, so H2 is paramagnetic. Bond dissociation energy in H2+ is 255 KJ mol-1, and bond length is 106 pm.
Molecular energy level diagram H2–:
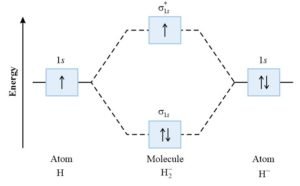
Bond order of H2– :
H2– means that the molecule of hydrogen gain one electron having configuration [ (σ1s)2 (σ*1s)1 ].
For H2– , bond order = 1/2 (2 – 1)=1/2 σ bond
Paramagnetic because it has one unpaired electron in ABMO (σ*1s)1.
Q: Which one is more stable H2, H2+, and H2–? Explain with reason.
Bond order is directly proportional to stability. The greater the bond order, the greater will be stability. H2 is more stable than H2+ and H2– because it has the highest bond order (1) than H2+ and H2– (1/2).
Q: Is the hydride H22- is exist? If not, why?
If the electrons in the bonding orbital and antibonding orbital are equal, the bond order is zero so, the molecule does not exist. H22- means the molecule of hydrogen gains two electrons. In case of H22-, bond order = 1/2 (2-2) = 0 no bond formation. Thus, this molecule doesn’t exist.
Frequently Asked Questions
What are bond order and its significance?
Bond order, which refers to the number of chemical bonds between a pair of atoms, shows how stable a bond is.
Significance of bond order:
- It aids in our comprehension of the bond’s stability.
- It aids in our comprehension of the bonding length and strength.
- We can tell if a molecule has been hybridized by looking at the bond order.
What is the bond order of H2(+)?
There is only one electron present in H2+.
- The (+) symbol denotes an electron loss.
- The only electrons in H2+ are bonding electrons.
- H2+ has a bond order of 0.5.
Is the bond of H2 polar?
The geometry of H2 is linear and the fact that both H-atoms have the same electronegativity, which causes them to participate in the same amount of charge, the H2 molecule is nonpolar with a net-zero dipole moment.
What does a 1.5 bond order mean?
A resonance hybrid would be produced when two or more potential Lewis structures for the molecule or ion were combined, hence a bond order of 1.5 would indicate the presence of resonance structures


Leave a Reply