
The solution has two types, the ideal solution, and the non-ideal solution. The ideal solution is a solution that follows Raoult’s law at all concentrations and all temperatures. To discuss in detail the study of ideal and non-ideal solutions, first, we understand what is the solution and how it is formed? The solution is a homogeneous mixture. Homogeneous means the properties of the solution are the same at all places or homogeneous means uniform properties. The solution is a homogeneous mixture of two or more substances that lose their individual identities and cannot be separated by a simple method. The solution consists of two-component one is solute (smaller amount) and the other is solvent (larger amount).
For example, 20% ethanol solution means 20% ethanol (solute) and 80% water (solvent). 80% ethanol solution means 80% ethanol (solvent) and 20% water (solute).
How Solution is Formed?

- Volume of pure water is V1
- Volume of pure vinegar is V2
- Volume of solution is Vf
- If Vf = V1+V2 then, ideal solution
- If Vf ≠ V1+V2 then, non ideal solution
Now we set a general example, let a pure liquid “A” have volume V1 and a pure liquid “B” have volume V2. On mixing of pure liquid “A” and “B”, the solution is formed having volume Vf.
ΔVmix
ΔVmix = Vf – (V1+V2) = change in volume on mixing
ΔHmix
- In pure “A”, A-A interaction is break so, heat is absorbed. (endothermic)
- In pure “B”, B-B interaction is break so, heat is absorbed. (endothermic)
- In solution “AB”, A-B interaction is formed so, heat is released. (exothermic)
ΔHmix = change in entholpy on mixing
Interactions between A-A, B-B and A-B
Is the interaction between A-A and B-B is equal to A-B or not?
For example, If the interaction between A-A and B-B is weak and interaction between A-B is strong. When the solution is formed the molecules come close to each other due to strong interaction between A and B and volume is decreases. If the volume is decreased then, ΔVmix is less than zero and the solution is a non-ideal solution showing negative deviation. For non-ideal solutions showing negative deviation:
Vf – (V1+V2) < 0
On the other hand, If the interaction between A-A and B-B is strong and interaction between A-B is weak. When the solution is formed the molecules are far apart from each other due to weak interaction between A and B and volume is increased. If the volume is increased then, ΔVmix is greater than zero and the solution is a non ideal solution showing positive deviation. For non-ideal solutions showing positive deviation:
Vf – (V1+V2) > 0
But if all the force of attraction between A-A, B-B, and A-B is the same then ΔVmix is zero and the solution is the ideal solution.
Vf – (V1+V2) = 0
ΔSmix
- ΔSmix = change in entropy on mixing
As entropy depends upon the number of particles so, the more the particles, the more the randomness, and more the entropy.
ΔSmix > 0 (always)
The ideal solution and non ideal solution do not depend upon ΔSmix because ΔSmix always increases.
ΔGmix
- ΔGmix = change in gibes free energy
As, if the process is spontaneous, the change in gibes free energy is less than zero.
ΔGmix < 0 (always)
The ideal solution and non-ideal solution do not depend upon ΔGmix because ΔGmix always decreases.
Raoult’s Law
- The solution which obey raoult’s law is ideal solution.
- The solution which do not obey raoult’s law is non-ideal solution.
Factors on Which Solution is decide, Which Is Ideal Or Non Ideal
- ΔVmix = Chnage in volume on mixing
ΔVmix = Vf – (V1+V2)
- ΔHmix = Change in enthalpy on mixing
- Interactions
A-A B-B = A-B
A-A B-B ≠ A-B
- Raoult’s law
Ideal Solution
- Ideal solution is a solution which follows Raoult’s law at all concentration and temperature.
PA ∝ XA
PB ∝ XB
Pobserved = P°A XA + P°B XB
Where,
- Pobs were obtained from experiment by the manometer.
- P°A XA + P°B XB was obtained from calculation by Raoult’s law.
If both the experimental and calculated values are the same then it obeys Raoult’s law and it is an ideal solution.
For Individual Components
PA = P°A XA (Ideal solution)
Where,
- PA were obtained from the experiment by the manometer.
- P°A XA was obtained from the calculation by Raoult’s law.
PB = P°B XB (Ideal solution)
Where,
- PB were obtained from the experiment by the manometer.
- P°B XB was obtained from the calculation by Raoult’s law.
2. ΔVmix = 0
Reason
“Due to same interaction”
A-A interaction = B-B interaction = A-B interaction then, ideal solution. The force of attraction between A-A, B-B, and A-B is the same. The forces neither increase nor decrease when the solution is formed. Due to the same force, molecules are neither closed to each other nor far apart so, the volume remains the same.
Vf – (V1+V2) = 0
3. ΔHmix = 0
Reason
“Due to same interaction”
- A-A interactions break so, heat absorbed (endothermic)
- B-B interactions break so, heat absorbed (endothermic)
- A-B onteactions formed so, heat released (exothermix)
If heat is absorbed during the breaking of A-A, B-B is equal to the heat released during the formation of A-B then ΔHmix = 0 and it is an ideal solution.
4. Force of attraction A-A = B-B = A-B
Graph For Ideal Solution
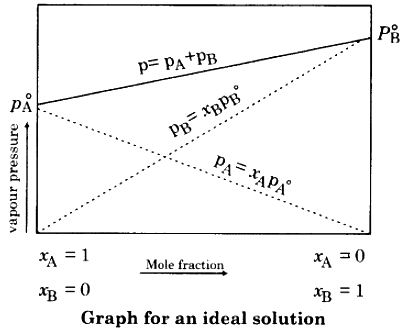
Examples of Ideal Solution
The force of attraction is the same when:
- Same polar nature
- Nearly same size
- CH3OH + C2H5OH
- C2H5Cl + C2H5Br
- n-hexane + n-heptane
- C2H4Br2 + C2H2Cl2
- C6H6 + C6H5CH3
Non-Ideal Solution
- Ideal solution is a solution which do not follows Raoult’s law.
Pobserved ≠ P°A XA + P°B XB
Where,
- Pobs were obtained from experiment by the manometer.
- P°A XA + P°B XB was obtained from calculation by Raoult’s law.
If both the experimental and calculated values are not the same then it does not obey Raoult’s law and it is a non-ideal solution.
For Individual Components
PA ≠ P°A XA (non -deal solution)
Where,
- PA were obtained from the experiment by the manometer.
- P°A XA was obtained from the calculation by Raoult’s law.
PB ≠ P°B XB (non-Ideal solution)
Where,
- PB were obtained from the experiment by the manometer.
- P°B XB was obtained from the calculation by Raoult’s law.
2. ΔVmix ≠ 0
Reason
“Do not the same force of interaction”
A-A interaction = B-B interaction ≠ A-B interaction then two possible outcomes:
- When the solution is formed, the force of interaction increase, molecules come close to each other, volume is decreased so, ΔVmix < 0.
- When the solution is formed, the force of interaction decrease, molecules far apart to each other, volume incresaed so, ΔVmix > 0.
3. ΔHmix ≠ 0
If heat is absorbed during the breakage of A-A, B-B is not equal to the heat released during the formation of A-B then ΔHmix ≠ 0 is a non-ideal solution.
4. Force of attraction A-A = B-B ≠ A-B
Deviations in Non-Ideal Solution
| Non-Ideal Solution Showing positive deviation from Raoult’s Law | Non-Ideal Solution Showing negative deviation from Raoult’s Law |
|---|---|
| Pobserved > P°A XA + P°B XB | Pobserved < P°A XA + P°B XB |
| ΔVmix > 0 | ΔVmix < 0 |
| ΔHmix > 0 | ΔHmix < 0 |
| A-A, B-B > A-B | A-A, B-B < A-B |
Non-Ideal Solution Showing Negative Deviation From Raoult’s Law
Why ΔVmix < 0 ?
Due to due to stronger interaction between A-B, molecules come close to each other and volume decrease.
Vf – (V1+V2) < 0
Where,
- Vf = Volume of A-B solution
- V1 = Volume of pure A
- V2 = Volume of purw B
Example
Let
- The volume of pure “A” is 20.
- The volume of pure “B” is 20.
When solution is formed, the volume of A-B solution is 30 due to stronger force of interaction between A-B than A-A and B-B. As,
ΔVmix = Vf – (V1+V2)
ΔVmix = 30 – (20+20)
ΔVmix = 30 – 40
ΔVmix = -10 < 0
Why ΔHmix < 0?
“Due to overall heat is released”
- ΔH = -ve mean overall heat is releaed.
- ΔH = +ve mean overall heat is obsorbed.
- A-A and B-B interaction is weak so less amount of energy is required or obsorbed to break bond.
- A-B interaction is strong so large amount of heat is relesaed during formation.
Heat released in formation of A-B > Heat obsorbed in breaking of A-A and B-B
Example of Non-ideal Solution Showing -ve deviation
- CHCl3 + CH3COCH3
- In chloroform, van der waal forces are present.
- In acetone, van der waal forces are present.
- When solution is formed, hydrogen bond.
2. CH3X + Oxygen, Nitrogen containg group
- CHCl3 + C2H5NH2
3. Strong acid + water
- HCl + H2O
- HNO3 + H2O
- H2SO4 + H2O
- HI + H2O
These examples showing negative deviations of non-ideal solution from Raoult’s Law.
Graph of Non-Ideal Solution Showing Negative Deviation From Raoult’s Law

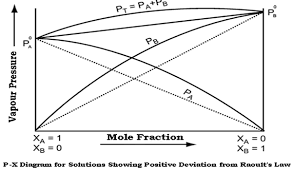
Non-Ideal Solution Showing Positive Deviation From Raoult’s Law
Why ΔVmix > 0 ?
Due to weaker interaction between A-B, molecules far apart and volume incresae.
Vf – (V1+V2) > 0
Where,
- Vf = Volume of A-B solution
- V1 = Volume of pure A
- V2 = Volume of purw B
Example
Let
- The volume of pure “A” is 30.
- The volume of pure “B” is 30.
When solution is formed, the volume of A-B solution is 80 due to weaker force of interaction between A-B than A-A and B-B. As,
ΔVmix = Vf – (V1+V2)
ΔVmix = 80 – (30+30)
ΔVmix = 80 – 60
ΔVmix = 20 > 0
Why ΔHmix > 0?
“Due to overall heat is obsorbed”
- ΔH = -ve mean overall heat is releaed.
- ΔH = +ve mean overall heat is obsorbed.
- A-A and B-B interaction is strong so large amount of energy is required or obsorbed to break bond.
- A-B interaction is weak so less amount of heat is relesaed during formation.
Heat released in formation of A-B < Heat obsorbed in breaking of A-A and B-B
Example of Non-ideal Solution Showing +ve deviation
- H2O + C6H6
- H2O + C6H5CH3
- CH3OH + C6H6
- C2H5OH + C6H5CH3
Graph of Non-Ideal Solution Showing Positive Deviation From Raoult’s Law



Leave a Reply