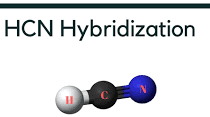
Before discussing the HCN hybridization we have to discuss how can we predict the geometry of the molecule using electron domains. Electron domains may include the VSEPR theory. The hybridization of any compound involves the intermixing of orbitals and forming of new orbitals before starting to explain the hybridization of HCN let’s have a look at what is HCN???
What is HCN??
HCN stands for hydrogen cyanide. It is considered to be a colorless, rapidly acting, and highly poisonous gas or liquid. Hydrogen cyanide is also called prussic acid. HCN acts as a weak acid due to strong intermolecular forces between hydrogen and cyanide.
So, it will be difficult for HCN to release its proton in an aqueous medium. It is a highly flammable liquid that can boil at a slight change in the room temperature of 25.6 degrees centigrade. HCN possesses three elements hydrogen, cyanide, and the most electronegative atom nitrogen.
Explaining properties of HCN:
There is a single bond between hydrogen and carbon but a triple bond between carbon and nitrogen in the cyanide group. HCN molecule has a bond angle of 180 degrees and has linear symmetry but it is a weak acid having a pka value of 9.2. As it is a weak acid so it has less degree of dissociation (∝).
HCN has some of the basic properties:
- Molar mass of HCN is 27.02 g/mol.
- The boiling point of HCN is 26℃ and its melting point is -13.29℃.
- It forms a homogeneous mixture with water and ethanol.
- It is a colorless liquid or gas.
- It has a density of 0.68 g/cm3.
- It has the odor of oil of bitter almonds.
What is Lewis’s structure of HCN??
Determining the lewis structure of HCN helps us to find the hybridization of HCN. Overall a neutral compound can form. This means a covalent bond is formed between an acid and a base. The following rules are under observation to draw lewis’s structure:
- The first step is to calculate the number of total valance electrons in the HCN molecule. Hydrogen has 1 electron, carbon has 4 electrons, and nitrogen has 5 electrons. Total electrons are 10.
H(1e) + C(4e) + N(5e) = 10 electrons
- The second step is to determine the central atom. The central atom is basically that atom that is less electronegative. just like HCN carbon is the central atom.
- Distribute all the ten electrons around the atoms
- Now in last, distribute the remaining electrons around the atoms.
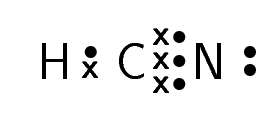
What’s about HCN hybridization???
HCN contains three elements in it. Hydrogen, carbon, and nitrogen. Before discussing the hybridization of HCN we have to discuss the hybridization between the CN. Cyanide will never exist in neutral form. It will exist in the form of CN-1. Cyanide will exist in the form of an unsaturated compound having a triple bond between carbon and nitrogen. If we discuss the electronic configuration of carbon and nitrogen.
C6 : 1s2, 2s2, 2px2, 2py0, 2pz0
C6*: 1s2, 2s1, 2px1, 2py1, 2pz1
N7 : 1s2, 2s2, 2px2, 2py1, 2pz0
N7*: 1s2, 2s2, 2px1, 2py1, 2pz1
In this case, overlapping occurs in the valance p orbitals of carbon and nitrogen. It will form px-px, py-py, and Pz-Pz overlapping outermost orbitals. In this compound, carbon has 4 half-filled orbitals and nitrogen has three half-filled orbitals. These orbitals overlap with each other.
But one orbital remains unpaired. This is the reason due to the presence of unpaired orbital in the neutral cyanide, it is not stable. So, in this regard to become a stable compound it may react with Hydrogen.
This means to say that Hydrogen requires only one electron to become stable and CN also requires only one electron to become a stable species. So, one electron of CN may react with the one electron of hydrogen present in the valance orbital. As a result, HCN is formed.
Hydrogen has only one electron in its outermost shell. So, its configuration is 1s1. It overlaps with the p orbital of carbon and forms sp hybridization.
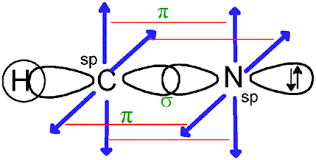
CONCLUSION:
Hence, by the above explanation, it is proved that HCN may possess linear geometry with a bond angle of 180o. And the bond formed between carbon and hydrogen is sigma because it is formed by head-on overlapping.


Leave a Reply