
The galvanic cell is also known as the voltaic cell or electrochemical cell which converts chemical energy into electrical energy due to a spontaneous redox reaction.
Why We Need the Galvanic Cell?
Before the galvanic or voltaic cell, an experiment has been done. By studying the experiment, you are able to find the answer to the question of why we need the galvanic cell?
Let us a container having an aqueous solution of CuSO4 which is blue in color and a zinc rod is placed in an aqueous solution of copper sulfate. The thermometer is also placed in a solution to measure the temperature. After some time, we observe some changes:
- Zn rod reduced in weight
- The blue color of the solution fades away
- Formation of red ppt. which is insoluble to solution
- Rise in temperature
What are the reasons behind these changes?
Oxidation of Zn
Zn(s) → Zn2+(aq) + 2e–
Reduction of Cu
Cu2+(blue) + 2e– → Cu(red ppt)
- Oxidation and reduction are occurring in the same container at the same time so it is a direct redox reaction.
- This is a spontaneous redox reaction.
- ∆G < 0
- The chemical energy is released in the form of heat.
But the problem is that we do not use this chemical energy to generate electric current the question is here what changes are done to generate an electric current from the chemical energy?
This question mark is the origin of an electrochemical cell or galvanic cell or voltaic cell.
What is Daniell Cell and its Function?
Daniell Cell is the Zn-Cu cell is an example of an electrochemical cell or galvanic cell or voltaic cell.
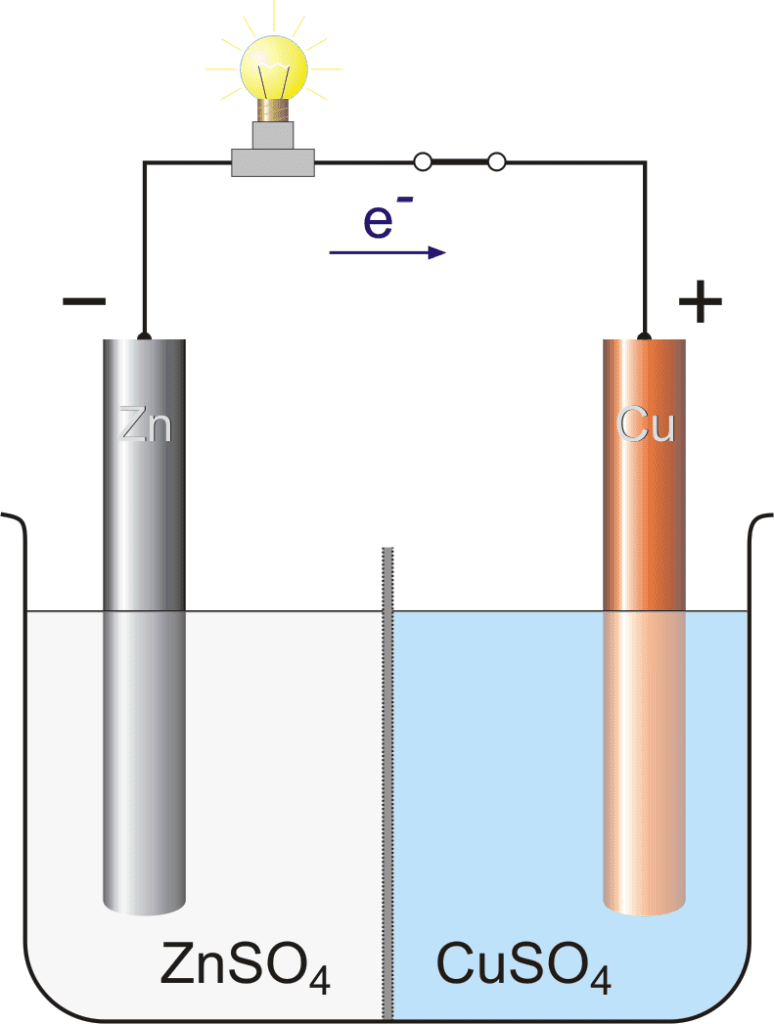
We have two half cells, the left half cell, and the right half cell. The left half cell consists of a Zn rod and the aqueous solution of ZnSO4. The right half cell consists of a Cu rod and the aqueous solution of CuSO4. Both the electrodes are connected with wire and an electrical bulb is also attached to detect the current.
In the left half cell, the spontaneous reaction occurs when Zn is oxidized and comes into the solution.
Zn(s) → Zn2+(aq) + 2e–
and the negative charge is deposited on the Zn rod. With the passage of time, these negative charge electrons which are deposited on the Zn rod is moves toward the Cu rod through the conducting wire.
In the right half cell, Cu2+ ions are present which want to reduce. The Cu2+ ions move toward the Cu rod and gain two electrons (that come from the Zn rod) and deposit on the rod.
Cu2+ + 2e– → Cu(s)
- Zn rod is a negative charge electrode due to the oxidation of zinc.
- Cu rod is a positive charge electrode due to the reduction of copper.
- The current flow from the positive charge electrode to the negative charge electrode. (opposite to the flow of electrons)

| Oxidation half cell | Reduction half cell |
| Left half cell | Right half cell |
| Negative half cell | Positive half cell |
| Anode half cell | Cathode half cell |
But there is a problem…!
In the left half cell, initially, the solution is neutral (equal moles of zinc and sulfates) but when zinc comes into the solution and a negative charge deposit on the zinc rod after some time this solution become a positive charge solution due to the high concentration of Zn2+ ions so the left half positive charge in solution prevents outwards flow of electrons. In the right half cell, initially, the solution is equimolar but with the passage of time the Cu2+ ions deposit on the copper rod, and the sulfate ions concentration increases. So the right half cell the negative charge in the solution prevents the inward flow of electrons. What is the solution to this problem?
Salt Bridge
A salt bridge is a solution to the above problem. It is inverted U-tube glass and the inert electrolyte is filled in U-tube glass. The electrolytes are KCl, H2SO4, KNO3, and NH4Cl in the agar-agar powder semi-liquid state.
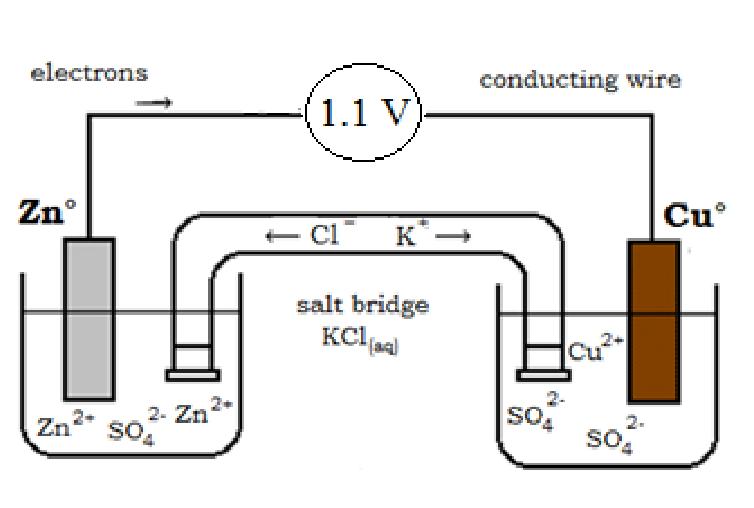
- The K+ ions move toward a negative charge solution in which the concentration of sulfate ions is greater.
- The Cl- ions move toward a positive charge solution in which the concentration of zinc ions is greater.
What is the function of the salt bridge in the electrochemical cell?
- It maintains the electrical neutrality of half cells.
- It completes the circuit by allowing the flow of electrons.
- It minimizes the liquid-liquid junction potential.
Conditions For Electrolytes that are Used in Salt Bridge
1) Electrolyte is Inert
The electrolyte is inert means the ions of the electrolyte do not react with ions or the rod of the half cell. For different galvanic cells, different electrolytes are used. In Daniell cell, KCl is used as an inert electrolyte in salt bridge.
KCl → K+ + Cl–
These ions do not react with any of the ions and rod of the half cell. It doesn’t mean these ions are completely inert. For example, the Cl– ions move toward the Zn2+ ion solution and form ZnCl2 but zinc chloride is a very soluble and strong salt. It quickly breaks down into its ions.
ZnCl2 → Zn2+ + 2Cl–
Similarly, K+ ions react with SO42- ions and form K2SO4 but it is also strong salt and completely dissociates into its ions.
K2SO4 → 2K+ + SO42-
If we have an Ag electrode, then we used KCl as an inert electrolyte?
We do not use KCl as an inert electrolyte because the Cl– ions react with silver ions and form white AgCl ppt which is insoluble.
2) Good ionic mobility and equal mobility of cation and anion
Due to the equal mobility of cation and anion, both half cells are neutralized simultaneously.



Leave a Reply