
Friedel-Crafts Reactions is a set of acylation and alkylation reactions. In this article, there is brief information about Friedel craft acylation and alkylation. Friedel Crafts reactions are types of electrophilic substitution reactions. These reactions are named after Charles Friedel and James Crafts. These reactions are of two types alkylation and acylation reactions. Friedel Crafts reactions are discovered in 1877 by Charles Friedel and James Crafts.
What are aromatic electrophilic substitution reactions?
Aromatic compounds give electrophilic substitution reactions in which an electrophile attack on the C-H bond resultantly the C-H bond breaks down and a new bond C-E (electrophile) is formed. This type of reaction is called electrophilic substitution reaction. The electrophile is an electron-deficient species and attacks the C-H bond.
These reactions are assisted by the addition of a catalyst.
The general mechanism of electrophilic substitution reaction is as follows:
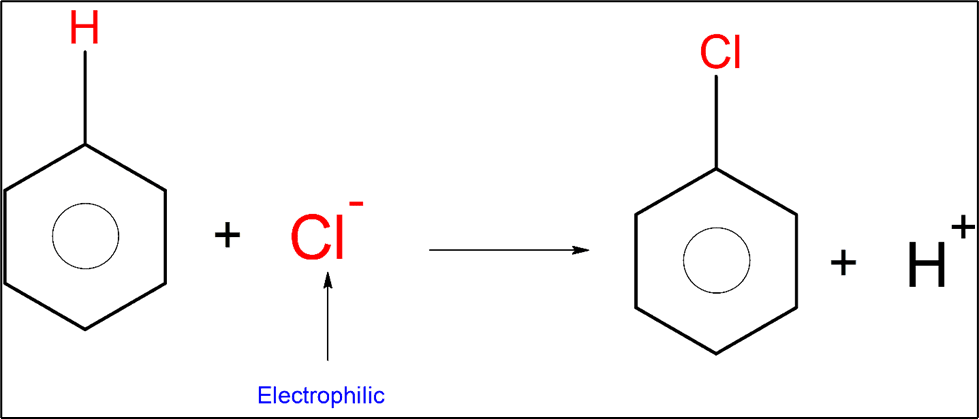
An example of an electrophilic substitution reaction is chlorination.
Friedel-Crafts Reactions:
Friedel craft reactions are a type of aromatic electrophilic substitution reactions. The Friedel craft reactions are of two types alkylation and acylation reactions. The most used version of the Friedel craft reactions is Friedel craft acylation.
Friedel -Craft Acylation:
The type of electrophilic substitution reaction in which the entering or coming electrophile( electron-poor species) is an acyl group RCO-, it originated from a carboxylic acid derivative and can be a halide or anhydride. In these acids, the carbonyl group is basic so that the formation of the complex takes place with the help of a strong Lewis acid such as Aluminum chloride.
- In this way, the carbocation character of carbonyl carbon is increased by adding aluminum chloride. But in many cases the complex formed itself is electrophilic and it reacts with an aromatic ring.
- In some cases, there is an equilibrium state when the complex exists in equilibrium with the acylium ion. That is a strong electrophile.
General mechanism of acylation:
The general mechanism for Friedel craft acylation reaction is the following. First of all the reaction of acylium ion with benzene is opposite to other electrophilic reagents. The final product is aromatic ketone in which the carbonyl group is basic and is complexed completely by aluminum chloride.
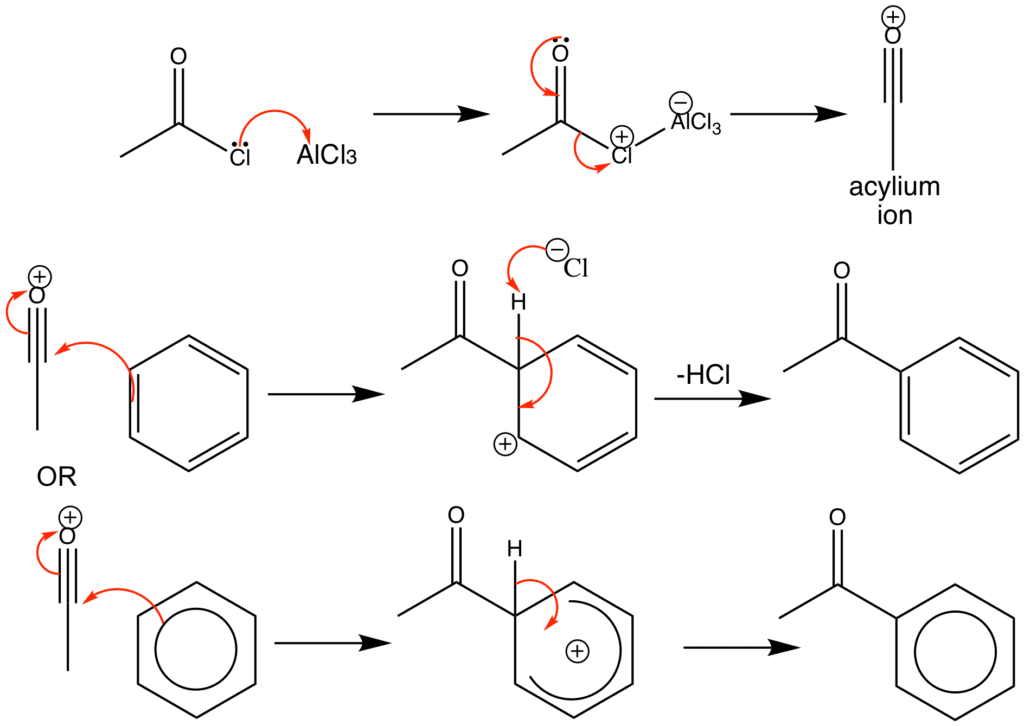
The complex that is formed is the actual reaction product. The workup procedure is involved treatment with water and diluting hydrochloric acid in this way the complex decomposes and aluminum salts are dissolved. Liberated ketone remains in the organic layer and it is isolated by crystallization and distillation. This is because it complexes with product and aluminum chloride must be used in equimolar amounts. And the complexed ketone does not react further and a high yield of the actual product is given readily.
In aromatic chemistry, the Friedel craft acylation reaction is an important electrophilic substitution reaction.
For example:
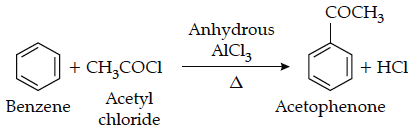
The reaction of acetyl chloride with benzene is an example of Friedel craft acylation reaction.
Limitations of Acylation:
- In acylation, the formyl-chloride decomposes into CO and HCl and only gives ketones.
- The aromatic compounds that are less reactive than mono-halobenzene do not participate in these reactions.
- Due to the formation of complexes the presence of deactivating groups on the aromatic ring can affect the working of Lewis acid catalysts.
Friedel-Craft Alkylation:
The type of electrophilic substitution reaction in which the coming electrophile (electron-poor species) is an alkyl group. Benzene undergoes an alkylation reaction with an alkyl halide in the presence of a Lewis acid catalyst.
The general reaction of alkylation is as follows.
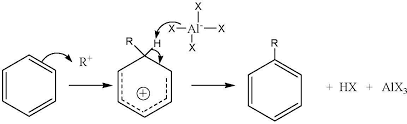
Reaction can be explained by putting the example of t-butyl chloride.
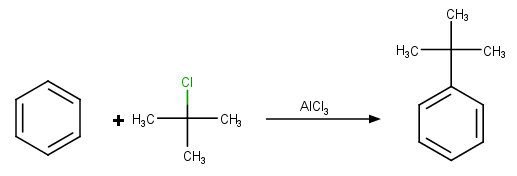
Mechanism of the reaction:
In the above reaction, the attacking electrophile is t-butyl chloride which is formed by the reaction of t-butyl chloride with FeCl3.
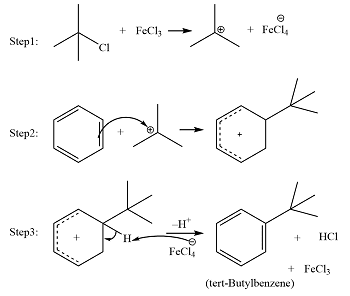
STEP 1:
In the First step electrophilic carbon is formed by reacting Lewis acid catalyst with an alkyl halide.
STEP 2:
And in the second step, the carbocation is attacking the aromatic ring resultantly a cyclohexadienyl cation intermediate is formed. In this way, the aromatic character of arene is lost temporarily because of the breaking of the C-C double bond.
STEP 3:
And in the third and last step deprotonation is occurred on the intermediate which results in again the formation of the C-C double bond. In this step, the aromatic character is again gained. And HCl or AlCl3 is also regenerated in this step.
Limitations of Alkylation:
There are limitations of Friedel craft alkylation so the Friedel craft acylation is mostly used version of Friedel craft electrophilic substitution reactions.
- Alkylbenzenes are more reactive than benzene itself in electrophilic substitution reactions. Alkylation reaction gives highly alkylated and di-alkyl products.
C6 H6 + (CH3 )3 Cl + FeCl3 →C6 H5C (CH3 )3
C6 H5C (CH3 )3 + (CH3 )3 Cl + FeCl3 → C6 H4 [C( CH3 )3 ]2
The way to control these additional reactions is to require benzene in large amounts. So this approach is practical with benzene itself and as this is an expensive compound. But this is impractical with substituted benzene which is more expensive.
2. Another limitation of Friedel craft alkylation reaction is that alternative reactions of a carbocation in the absence of a particular nucleophile lead to rearrangement of isomeric carbocations. Isopropyl chloride reacts normally with benzene to give isopropyl benzene.
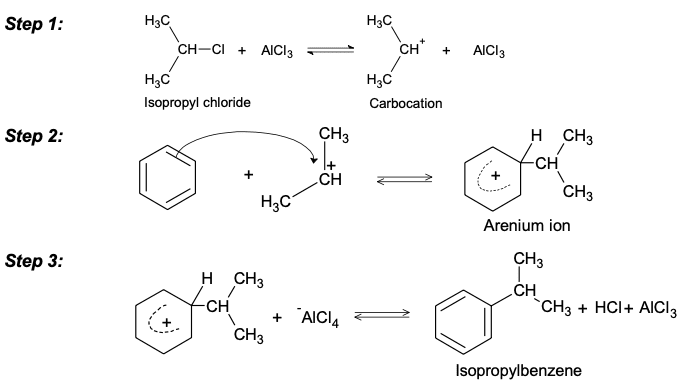
Primary alkyl-halides are less reactive than secondary or tertiary halides and require higher temperatures. Under some conditions, there is a partial rearrangement of the primary system. So in this case alkyl halide on benzene coordinate a displacement reaction with Lewis acid to compete for the carbocation rearrangement. It should be emphasized. But rearrangements occurs always with the primary system. So this limits the usefulness of this reaction.
3. Friedel craft alkylation reactions are also given by alcohol with the Lewis acids aluminum chloride and boron tri-fluoride. But here is a limitation to this reaction that these reactions require benzene excess as in the case of alkyl halides. And one of the products of this reaction is water that can coordinate with Lewis acid. And therefore in the case of alkylation reactions the Lewis acid is also required in a stoichiometric amount.
Friedel -Craft Alkylation by Alkenes:
Friedel craft alkylation is also given by alkenes. The catalyst typically used in the case of alkenes are HF, BF3, and HCl or AlCl3.
An example of this type of reaction is:
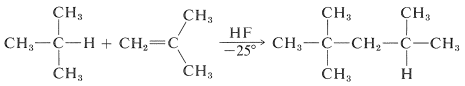
This type of reaction has an industrial use to prepare alkylbenzenes.
Which group will give Friedel-Crafts Reactions easily?
The organic compounds that have electron-donating groups give Friedel craft reactions easily. While the nitrobenzene does not give Friedel craft reaction due to the strong withdrawal group the nitro group is attached to it.



Leave a Reply