
What are the factors affecting reaction rates and why? In this context, we detailed discussed the factors that influence the rate of reaction. The rate of reaction means the change in concentration of reactant or product with respect to time. Basically, there are two factors on which the rate of reaction depends:
- Concentration
- Temperature
But we will discuss also some other important factors including concentration and temperature.
What are the Factors Affecting Reaction Rates and Why?
1. Nature of Reactant
What the nature of the reactant is? There are three types of reactants:
Ionic Reactants
The reactant is ionic in nature like NaCl, AgNO3, etc.
NaCl + AgNO3 → AgCl ↓ + NaNO3
The rate of reaction of ionic reactants is very high.
Covalent Reactants
The reactant is covalent in nature means the bond formed between the atoms is due to the mutual sharing of electrons. like nitrogen N2, hydrogen H2, carbon dioxide CO2, water H2O, etc.
N2 + 3H2 → 2NH3
The rate of reaction of covalent reactants is very low.
Homogeneous and Hetrogeneous Reactants
Homogeneous reactants mean having the same size of an atom, the same size of molecules, and the same force of attraction. Heterogeneous reactants mean having different sizes of atoms and molecules and also the force of attractions. So the rate of reaction of homogeneous reactants is greater than heterogeneous reactants.
Rate of Reaction is Directly Proportional to the Stability of Product
Rate of reaction ∝ Stability of the product
Rate of reaction ∝ 1 / Stability of the reactant
2. Physical State of Reactant
The order of rate of reaction is given below:
Gases > Liquid > Solid
3. Surface Area of Reatant
The rate of reaction is directly proportional to the surface area of the reactant.
Rate of reactant ∝ Surface area of the reactant
Let us have sugar cubes and sugar powder, which one is easily soluble in milk?

Sugar powder is more soluble in milk as compared to sugar cubes because sugar powder has a large surface area to dissolve.
4. Intensity of Light
As we know that some reactions are photosensitive (photochemical).
H2 (g) + Cl2 (g) → 2HCl
If we increase the intensity of light, it means the number of photons increases.
Intensity of light ↑ = no of photons ↑ = Rate of reaction ↑
5. Catalyst
The catalyst speeds up or down the rate of chemical reactions. The catalyst sets up an alternate path from reactants to products with lower activation energy.
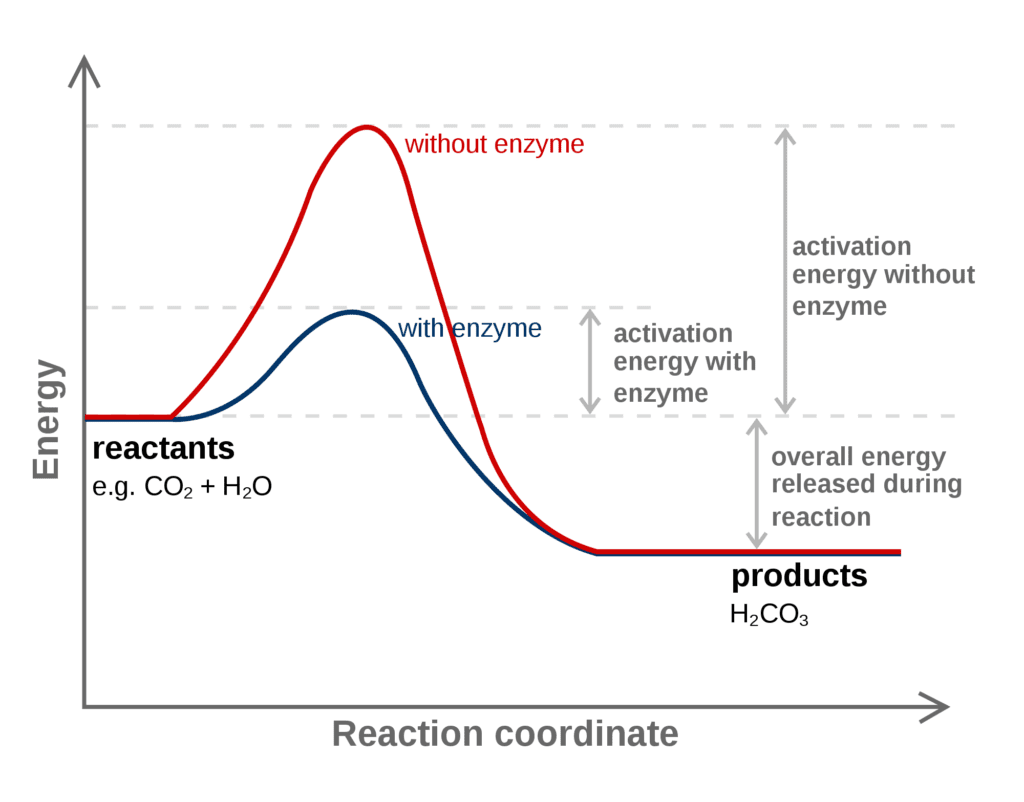
For example, in a given reaction, SO2 + O2 → SO3 the catalyst used is nitric oxide, and experimentally seen that the rate of reaction is directly proportional to the concentration of nitric oxide.
Rate of reaction ∝ [NO]
6. Temperature
Generally, the rate of reaction increases with increasing temperature. At a 10°c rise in temperature, the rate of reaction increases two to three times.
7. Concentration of Reactant
Generally, the rate of reaction increases by increasing the concentration of the reactant.
- The rate of reaction may also decrease on increasing the concentration of reactants.
- The rate of reaction remains unaffected.
- The rate of reaction may also depends upon the concentartion of product.
- The rate of reaction may depend on one or all or none of the reactants concentration.

So all these are the factors affecting reaction rates.



Leave a Reply