
This blog provides complete and useful information about the electron domains, and how electron domains are involved in determining the geometry of the molecule. Electron domains play an important role in the prediction of molecular shape and dimensional arrangement of molecules. So, VSEPR theory helps us to predict the geometry of molecules but before VSEPR, the shape of molecules is predicted by the Lewis structure. To understand the prediction of geometry we have to understand the concept of lone pair and the different bonds formed between the two atoms.
This will help us to understand the concept of VSEPR theory. In chemistry, the electron domain is defined as “the number of lone pairs and bonds surrounding the central metal atom is called electron domain“. It is also known as the electron group. Following are the key points that are related to the electron domains:
- The number of lone pairs and bonds surrounding the central metal atom is called the electron domain that is used to predict the geometry of the molecule.
- Prediction of a geometry occurs due to the distribution of electrons around a central metal atom to minimize and reduce the maximum repulsion between another atom.
- Due to the same charge nucleus and positively charged particles repel each other but opposite charges i.e. electrons and positively charged nuclei attract towards eachother. So, the repulsion of electrons is not only the factor that describes molecular geometries.

What is Lone pair?
A lone pair is associated with the negative charge. A pair of electrons that is not reacted or shared by an atom is called lone pair. It is connected to an atom by using a coordinate covalent bond. It is also called a non-bonded electron pair or unshared pair of electrons. We can identify the lone pair by using the Lewis structure. To calculate the number of valance electrons in an atom, we add lone pair in the bonding electron pair.
Valance electrons in atom = Lone pair + Bond pair
The lone pair is involved in determining the geometry and shape of the molecule, and it is an essential part of the electron domain.
- A lone pair is involved in changing the angle of the molecule. Therefore, the lone pair exhibits a polar character. The presence of lone pair decreases the bond angle because of higher electronic repulsion and higher electrical charge between the lone pair and the bonding pair of electrons. Further illustration occurs in VSEPR theory.
- A lone pair is also used to create a dipole moment. For example in NH3: a single lone pair is present on the nitrogen atom which exhibits a negative character but hydrogen exhibits a positive character. So, both exhibits opposite character and does not cancel out eachother effect so possess a dipole moment.
- By changing the angle due to lone pair the geometry and shape of the molecule change. Before VSEPR, lewis structure is used to predict the shape of the molecule.
Prediction of geometry/shape (VSEPR Theory)
VSEPR stands for the Valance Shell Electron Pair Repulsion Theory. VSEPR was first presented by Sidgwick & Powell in 1940. VSEPR is based on the premises that there is repulsion between lone pair of electrons and always atom will arrange itself in that manner where there is minimum repulsion. This arrangement determines the geometry and shape of the molecule as well as the bond angle. So, in VSEPR complete electron domains predict the complete geometry, shape, and bond angle.
Postulates of VSEPR
Postulates of VSEPR are given below:
- A lone pair occupies more space then a bond pair. This is due to the lone pair of electrons is under the influence of only one nucleus of the central metal atom, they are expected to occupy with a greater electron density ten the bond pair electrons which are under influence of two nuclei. The decreasing order of repulsion is:
lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
- Repulsive forces decrease very sharply with increasing interpair angle. They are stronger at 90 degrees, much weaker at 120 degrees, and very weak at 180 degrees.
- The influence of a bonding electron pair decreases with the increase in the electronegativity of an atom forming the molecule.
- Multiple bonds behave like the single electron pair for the purpose of VSEPR theory.
- Two electron pairs of the double bond or three electrons of the triple bond occupy more spaces than one electron pair of a single bond.
- The lone pairs repel bond pairs giving rise to some distortion in the molecular shape. The distortion may also result due to different atoms in the molecule.
Electron Domain Geometries
Electron domain geometries may include five shapes that a molecule possesses, i.e. linear, tetrahedral, Trigonal planar, Trigonal bipyramidal, and octahedral. There are many exceptions in the geometries as well as the shapes of molecules. They may be square planar, bent, T-shaped, etc. These all are based on the arrangement of lone pairs (Electron domains). Their explanation is given below in the form of the table:
| GEOMETRIES | PROPERTIES |
| 1. Linear | It is an arrangement of 2 electron domains. The outer atoms are at 180o from eachother. The geometry lies in a straight line. |
| 2. Trigonal Planar | It is an arrangement of 3 electron domains. At corners, three atoms lie in the equatorial plane. All atoms are at 120o from each other. |
| 3. Tetrahedral | It is an arrangement of 4 electron domains. It has 4 sides or faces and is 3D geometry. All the atoms are at 109.5o from each other. |
| 4. Trigonal bipyramidal | It is an arrangement of 5 electron domains. In this two plates developed. The atoms in the equatorial plate are at 120o and the atoms at the axial plate are at 90o. |
| 5. Octahedral | It is an arrangement of 6 electron domains. In this, all atoms are at 90o from each other and due to symmetry between them, it does not occupy any axial or equatorial plate. |
How to explain the geometry or shape of molecules?
Explanation of geometry is a very essential application of VSEPR theory. We can explain the geometry of the molecule in the following ways. First, to determine geometry we draw the lewis structure. The second step involves the determination of electron pairs (lone pair and bond pair). In the last step, we determine the number & location of the lone pair of molecules.
Gillespie Proposed some of the following rules that can explain the shape of the inorganic molecules:
- Rule No. 1: If the central atom of a molecule is surrounded only by bonding electron pairs and not by non-bonding electron pairs is called lone pair, the geometry of a molecule will be regular, example it will be linear, triangular planar, tetrahedral, trigonal bipyramidal, and regular octahedron for 2, 3, 4, 5, and 6 bonding electron pairs.
- Rule No. 2: When the central atom in a molecule is surrounded by both bond pairs and lone pairs the molecule does not have a regular shape. The alternation or distortion in shape is due to the alternation in bond angles which arises due to the presence of lone pairs on the central atom.
- Rule No. 3: B-A-B bond angle decreases with the increase in electronegativity of atom B in the AB molecule where A is the central atom this is due to the fact that with the increase in electronegativity of atom B. The average position of the bonding electron pair moves further from the central atom A and hence the repulsion is exerted by bonding electron pairs on the electron pair on atom A decreases. The decrease in repulsion decreases the bond angle.
- Rule No. 4: Bond angles involving multiple bonds are generally larger than those involving only single bonds. However, the multiple bonds do not affect the geometry of a molecule.
- Rule No. 5: Repulsion between electron pairs in filled shells is larger than the repulsion between electron pairs completely.
Complete Explanation of shapes/geometries
1. Linear geometry:
A molecular geometry in the field of chemistry describes the bond angle of 180o. In this geometry, two ligands are attached on both sides of the central metal atom. Due to the maximum repulsion between both of these ligands both are pulled apart from each other and result in the bond angle of 180o. Due to the same functional group or ligands on both sides, they cancel out each other effect and hence have no dipole moment. It possesses a dipole moment of 0.
Linear geometry may also occur if the central metal atom may possess two or three lone pairs. In this situation, the AXE notation for linear geometry is AX2 & AX2E3.

2. Trigonal Planar
- There are two geometries related to Trigonal planar. One is triangular and the other is bent. Triangular is the geometry in which the central metal atom of a molecule is surrounded by the three atoms and due to repulsion, the bond angle becomes 120o. The presence of ligands or atoms makes the structure just like an equilateral triangle. The ideal triangular geometry may possess all three atoms identical.

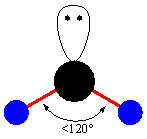
- The next type related to Triangular planar is Bent. It is the geometry having a single lone pair on it and having two identical atoms. There is minimum repulsion between the atoms and lone pair. So, it possesses the bond angle less then 120o. It is a molecular non-collinear arrangement having V-shape. In this, the angle does not go less than 90o.
3. Tetrahedral:
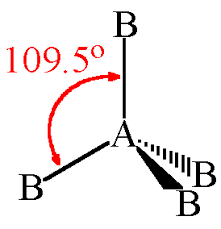
Tetrahedral geometry is the geometry in which four ligands or atoms are attached to the central metal atom and have no lone pair. This structure occurs in a 3D plane. In this structure, four substituents are attached at the four corners of the tetrahedron forming the bond angle 109.5o.
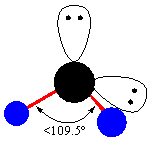
- The other shape related to the tetrahedral geometry is the trigonal pyramidal. This geometry possess one of the lone pair. The factor of repulsion may cause a decrease in bond angle. So, it has a bond angle of less than 107.5o. It resembles tetrahedral geometry because a lone pair is present on the apex and three are present on the base of the central metal atom.

- Another form of Tetrahedral geometry is bent. In this geometry, a central metal atom may possess two lone pairs and two atoms or ligands. Due to repulsion between lone pair and atom the bond angle decreases. It possesses a bond angle of less than <<<109.5o.
4. Trigonal Bipyramidal
- Trigonal bipyramidal is the shape in which the molecule has one central metal atom and five atoms surrounding the central metal atom. In this structure, two planes are formed, i.e, the Axial and equatorial planes. At the equatorial plane, the atoms are at 120oof each other and the atoms at the axial plane are perpendicular to each other at 90o.

- The second shape related to the trigonal bipyramidal is sawhorse. In this geometry, there formed two planes axial, and equatorial. But there is some of the dissimilarity between trigonal bipyramidal, and sawhorse geometry. The presence of lone pair in sawhorse geometry makes it dissimilar from trigonal bipyramidal. There is a great difference in bond angle. Presence of lone pair decreases the bond angle <120o and <90o.
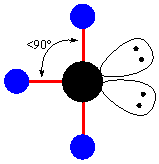
- The third geometry related to the trigonal bipyramidal is T-shaped. In this structure, there is presence of two lone pairs and three atoms or substituents. These three atoms are attached to the central metal forming the bond angle at axial plane less then 90o (<<<90o).
- Forth geometry related to trigonal bipyramidal is linear. In this geometry, 3 lone pairs are present which can increase maximum repulsion and brothers possess no bond angle. There are two ligands or substituents attached to the one central atom at the axial plane at which the bond angle becomes 180 degrees.
5. Octahedral
- Basically, in octahedral geometry, the central metal atom is combined with the six atoms or ligands. At the axial plane, there should be 2 atoms, and at the equatorial plate, there must be 4 atoms attached to the central metal atom. The bond angle between the axial and the equatorial plane will be 90o.
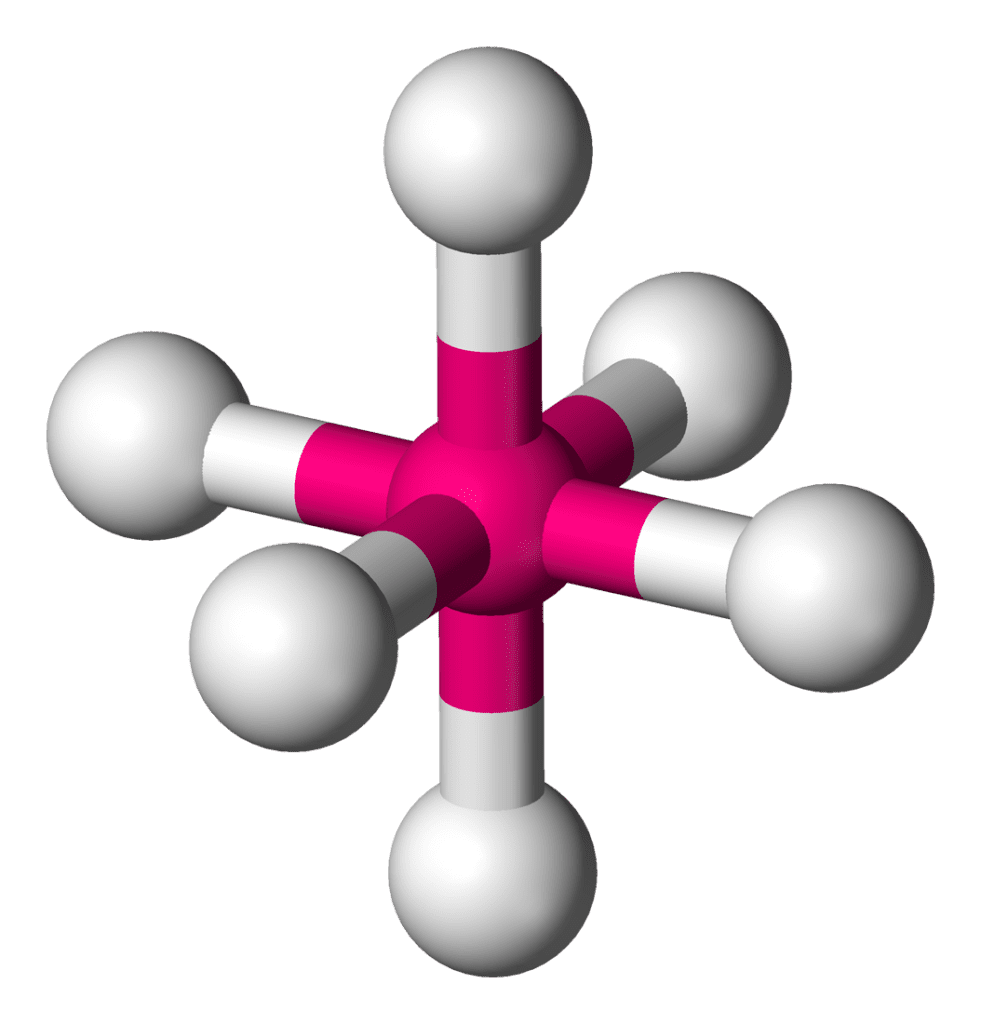
- All other types related to the octahedral geometry are the square planer, Linear, T-shaped, and Square pyramidal. There is no any difference between all these kinds. But some of the dissimilarity occurs due to the increase in number of lone pairs. Therefore, an increase in number of lone pairs causes an increase in repulsion and a decrease in bond angle.
Limitations of VSEPR
Following are the limitations of VSEPR:
- VSEPR theory does not explain the shpe of molecules having polar bonds.
- Shapes of molecules having delocalized pie electron systems are not explained.
- Shapes of compounds having inert pairs of electrons are cannot be explained by VSEPR.
- Some transistion metal complexes’ shapes cannot be predicted by VSEPR.
Conclusion
In this blog, we will discuss in detail the concept of electron domains, lone pairs, and how this electron domain is involved in predicting the shape and geometry of the inorganic molecules.


