
The sample container or cuvette that contains the analyte which is to be analyzed is the third most important component of the spectrophotometer.
What is a Glass Cuvette?
The sample container or cuvette should be transparent in the spectral region of interest.

This can be used for visible spectrophotometry but not for UV due to the impure form of glass. In visible spectrophotometry, we analyze the colour solutions. Colour solutions mean these solutions show absorption from 400 to 800 nm that’s why glass cuvette is used. As in a glass, there are impurities like silica, any organic compounds, and there will be a chance that some substances present in glass don’t absorb some colours. The double and triple bond compounds usually observed the UV light and if these compounds are present in the manufacturing of glass (as we say, glass has many impurities) so we do not use glass cuvette for UV spectrophotometry.
And another point is that plastic cuvette can also be used for visible spectrophotometer but not for UV because plastic has also many impurities. For UV spectrophotometry, one thing is must that cuvette material must not have any double or triple bond species. It is because UV radiations are mostly used for the analysis of an analyte to know that analyte has either double bond or triple bond.
Why do we use Quartz Cuvette?
Quartz is a pure form of silicon dioxide, SiO2 that is single-bonded specie and is suitable for UV spectrophotometry. As it does not have any double bond so will not absorbed in the UV region of the spectrum.

The front and back side are transparent and the left and right sides of the quartz cuvette are non-transparent. These are more expensive than the glass cuvette. Whenever we have to put it in spectrophotometry, we touch its non-transparent sides, the transparent sides should be clear and should be placed in the path of light beam.
Are all Cuvettes the Same Size?
Cuvettes are available in various sizes or shapes. The most common is 10 mm size however if the solution to be analyzed is very dilute then we can use a cuvette of large size. The “millimeter” is used or represent the diameter of the cuvette. If we use a large cuvette, absorption or emission signal is more for example if you use a 100mm cuvette, in the path of light more particles absorb light so there is more absorption. The diameter of the cuvette is the path length of the beam. If you have a concentrated sample solution then you have to use a 10 mm cuvette but if you have a dilute sample solution then you have to use a large size cuvette in order to get the measurable unit. But sometimes there is any instrumental problem that in some instruments there is a universal setting that where we place cuvette, we can increase or decrease the area of that place in order to fix the cuvette of any size, but some instruments have fixed size so we have to insert particular size cuvette.
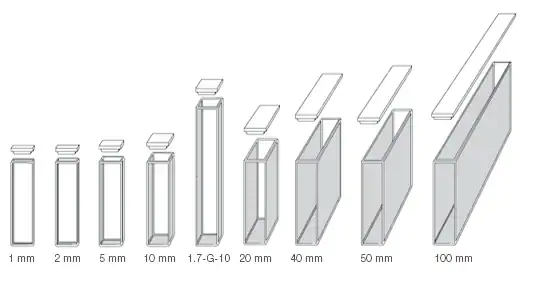
How does the Size of a Cuvette Affect Absorbance?
The diameter of the cuvette is the path length of the beam.

The greater the diameter, the greater will be the number of molecules that will strike or come in the way of incoming electromagnetic radiation, and the greater will be absorption or emission signal.
Path length ∝ Absorption or emission
For concentrated sample solution, smaller diameter cuvettes are used in order to get optimum results.
What is a Micro Cuvette?

Sometimes the sample solution is in a very less amount to fill the whole cuvette volume. In that case, a cuvette having a channel inside its body is used. These are called micro cuvettes but have the same path length of 10 mm.
Reflecting Walls Phenomenon
For very dilute solutions, sometimes a special type of cuvette is used having reflecting walls. The beam of light or electromagnetic radiation travels back and forth many times before it exists in the sample container. In this way, we get a measurable absorption or emission signal for very dilute solutions. This strategy is applicable only to those analytes that absorb less light.
Which Cuvette is used in Spectrophotometer for Analysis in IR region?
In IR, the sample is needed in less amount, or in IR we analyze double, single, or triple bonds means cuvette material must not be of double, single, or triple bonded (means no covalent bond). For IR Spectroscopy, cuvettes are made of NaCl or KCl crystals. These are very expensive and delicate to use. These cuvettes are used as a sample container or as a substrate or glass slides means sometimes we just paste the sample on these slides and strike the infrared radiations. As NaCl or KCl is ionic in nature so all absorption is due to the sample analyte, not due to the sample container. Since in IR, we use we are expecting all sorts of single, double, or triple bonds, so cuvettes are made up of material that does not have these bonds.
How do you Insert a Cuvette into a Spectrophotometer?
It is necessary to clean the sides of the cuvette in order to remove any fingerprints that may affect absorption or admission reading. The cuvette is filled only 3/4 of its size, overfilling is avoided in order to stop spillage inside the instrument. Most instruments have the beam of the light source or electromagnetic radiation passing through the bottom portion of the cuvette. So it is safe not to fill the cuvette completely but should be 3/4 parts only.



Leave a Reply