
Chromatography is a method of separation used for qualitative and quantitative analysis. Just as atomic spectroscopy or molecular spectroscopy chromatography is also a full-fledged technique used in the analysis of samples.
Chromatography is a separation technique. This technique was introduced by Russian Botanist Mikhail Taswett by separating plant extracts by using petroleum ether as the mobile phase or calcium carbonate as a stationary phase by using the procedure of column chromatography.
Definition:
Chromatography is the sum of two words chrome means color and graphy means to write. It is a physical method of separation in which components to be separated are distributed between two phases. One of these two phases constitutes a stationary phase of a large surface. The other is a mobile phase which may percolate along or through the stationary phase.
Column chromatography works under the influence of gravity. Twswett uses calcium carbonate as a stationary phase. By loading sample in a column and mobile phase run under the influence of gravity. As the mobile phase runs we will observe different content appear. AS shown in the diagram how the content will appear in different colors. This technique was called chromatography because bands of different colors appeared.
Modification of Chromatography:
Later on, this technique was further developed by the Synge or Martin. They won a noble prize in 1952. Now chromatography is a full-fledged technique for both qualitative and quantitative analysis.
Mobile phase:
The extracting phase in chromatography that moves through the system is called the mobile phase.
Stationary phase:
The extracting phase in chromatography that remains in a fixed position is called the stationary phase.
Types of Chromatography:
There are the following types of chromatography.
- Column chromatography.
- Paper chromatography.
- Adsorption chromatography.
- Partition chromatography.
- Ion exchange chromatography.
- Size exclusion chromatography.
Column Chromatography:
Column chromatography works under the influence of gravity. In this type of chromatography, a column is used in which there is an opening at the lower side stopper is present also. Through opening material move down.
The mobile phase percolates or moves along the stationary phase. As the mobile phase moves different content appears.
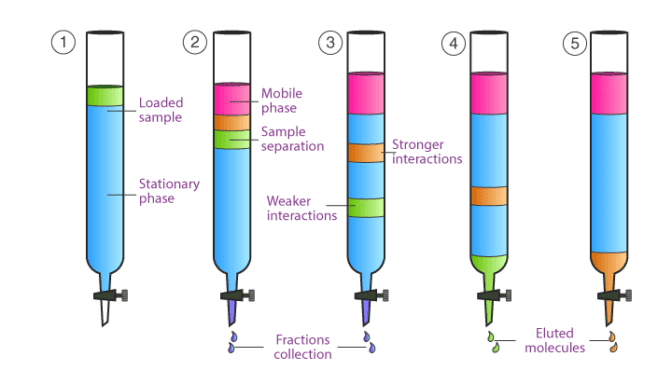
As seen in the diagram that when the sample is loaded. After adding the mobile phase different content appeared. One of the content is in brown color and the other is green. Original content changes to different colors this is due to the percolation of the mobile phase through the stationary phase. The content that has more interaction with the stationary phase moves slowly. The content that has interaction with the mobile phase moves fast through the column and has less interaction with the stationary phase. As with the passage of time, the slow-moving content also separates from the column. This is called elution and content is called eluted content.
This is all about the basic principle of column chromatography.
Adsorption Chromatography:
In this type of chromatography, the stationary phase is solid whereas the mobile phase is liquid or gas.

As in this diagram, we can see that solute particles are adsorbed on the surface of the stationary phase. The particles are the analyte and dissolved in the mobile phase showing interaction with the stationary phase.
Thin-layer chromatography (TLC) :
Thin-layer chromatography is a type of adsorption chromatography. It is the same as paper chromatography. In thin-layer chromatography, just in place of paper, there is a glass plate on which the stationary phase is coated and this is just like the paper dipped in the mobile phase. The mobile phase passes through it. The stationary phase is solid and the mobile phase is liquid here. There occurs physical adsorption between the mobile phase and stationary phase.

Partition Chromatography:
In partition chromatography both phases are the same, either liquid or gas there is no difference in phases. Partition of components between two phases occurs.
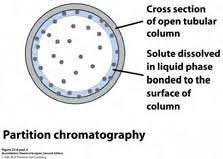
As shown in the diagram it is a tubular cross-section. The solid surface is coated with liquid which is called the stationary phase. The mobile phase is also liquid. There is a partition of components between these two phases.
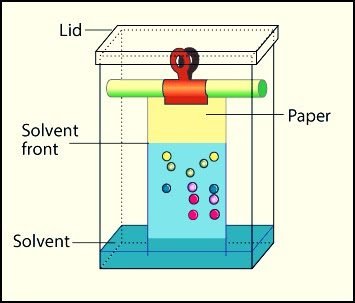
Paper Chromatography:
Paper chromatography is the type of partition chromatography. As the paper is dipped in solvent. Paper is made of cellulose which is 90% water. So stationary phase is also liquid and both phases have the same physical state. There is a partition of components on paper. The mobile phase moves through the paper.
Size Exclusion Chromatography:
It is also called molecular exclusion. In this type of chromatography, separation is based on the size of molecules or components.

As shown in the diagram there is a network on the surface of the stationary phase. Only small molecules can pass through this network large molecules will not pass. Large molecules exclude. That is why it is called molecular exclusion chromatography. Excluded molecules move down. Small molecules having high concentrations outside these move inside the stationary phase where their concentration is less.
When a fresh mobile phase is added the small molecules will move outside and elute out. As shown in the column diagram actually stationary phase is in the form of jell balls that are porous. Small molecules move inside porous balls. Large molecules remain outside and elute out directly. After adding a fresh mobile phase small molecules also elute out on the basis of concentration.
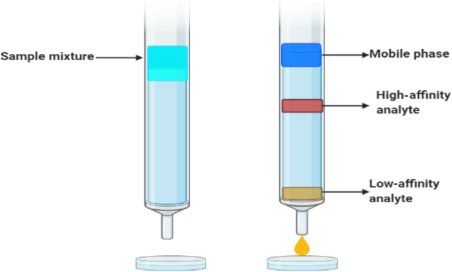
Affinity chromatography:
It is a specific type of chromatography that is not commonly used. This type of chromatography is used for antigens, antibodies, or other biological structure separation. In this type of chromatography, cavities are formed on the surface of the stationary phase. Particular shape molecules can attach to a particular cavity. The remaining will elute out. This work like lock and key.

Ion Exchange Chromatography:
This is a special type of chromatography used for the separation of ions on the basis of their interaction with stationary phases.
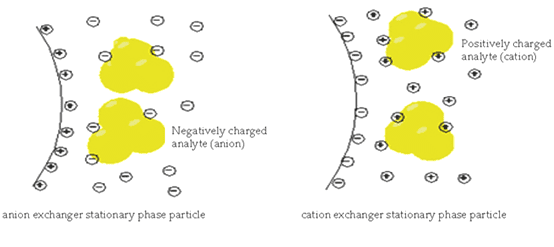
In anion exchange chromatography in the stationary phase, there is the grafting of positive ions that are covalently attached to the surface of the stationary phase. And the anions from the analyte have some interaction with these positive ions present in the stationary phase. The anions having less interaction with these cations move fast downward whereas those having more interaction having more charges will strongly bond with positive ions of the stationary phase. In this way on the basis of charges interaction separation of ions occur.
If there is the grafting of negative ions in the stationary phase then the analyte having positive ions will attract towards the stationary phase. Cations having more interaction will hold strongly to the stationary phase. And cations having less interaction with the stationary phase will pass through. In this case, there is cation exchange occurs.
Overall this is called ion-exchange chromatography.



Leave a Reply