
Chiral center is defined as an atom bonded tetrahedrally to four different groups or atoms called ligands. Chiral center usually C atom but may be N, S, P, Si etc. . In other words if we define chiral so we can say any object is said to be chiral if it is different than its mirror image. Means to say it is non-superimposable on its mirror image. On the other hand achiral objects are superimposable on its mirror image. In the given content there is brief discussion on chiral center and how we can identify chiral center in compounds.
Two enantiomers of amino acid are chiral as shown in figure.

Chirality:
Chirality is a stereochemical property. A molecule is chiral if it is non-superimposable on its mirror image. If a molecule is chiral it contains at least one chiral center.
Enantiomers:

When a molecule is chiral , it will have two isomeric forms called enantiomers. These are non-superimposable mirror image of each other.
- Enantiomers are stereoisomers .
- They have same molecular formula and sequence of bonded elements.
- They have different spatial arrangement of groups in molecule.
Chiral center:
Chiral center is an atom bonded tetrahedrally to four different groups or atoms called ligands. Chiral center usually C atom but may be N, S, P, Si etc.
- In organic compounds, chiral center is an asymmetric tetrahedral carbon atom that is bonded with four different groups. Chiral molecules have two enantiomers and at least one chiral center.
- When a molecule contains a chiral center than it lacks symmetry elements thus chiral. It contains two enantiomers that are non superimposable mirror images of each other.
- The four different groups attached to a chiral carbon atom may be different elements, isotopes, functional groups.
- Chiral center is present in both open chain and cyclic compounds.
Stereogenic center:
A tetrahedral carbon atom with four different groups attached is also a stereogenic center.
A stereogenic center is an atom at which the interchange of two ligands results in stereoisomers.Means that interchange of two groups bonded to chiral carbon results into stereisomers of that molecule.If there is a single chiral center then interchange of two groups results into enantiomers of that molecule.
Configuration of Chiral center:
The recognition or determiniation of chirality and chiral center is an important step to determine the number of stereoisomers that are possible for a given compound.
If a single chiral center is identified in a molecule, then there are two stereoisomers of that molecule. Stereoisomers(enantiomers) differ in the spatial arrangement of groups around the chiral center. Enantiomers are distinct isomers. To identify enantiomers we nominated the spatial arrangements of groups around the chiral center. Early method to designate enantiomers as either L or D based on their relationship of chiral center to chiral center in D- or L-glyceraldehyde. But there is a problem in this system of nomenclature that is it is difficult to apply this to larger molecules, especially those containing more than one chiral center.
In response to early system of nomenclature IUPAC (International Union of Pure and Applied Chemistry) system of nomenclature was developed. And now this is a standard method used for the configuration of chiral center in a molecule. Each of chiral center has two possible mirror image configurations. That is designated as R or S configuration.
R’ or S’ Configuration:
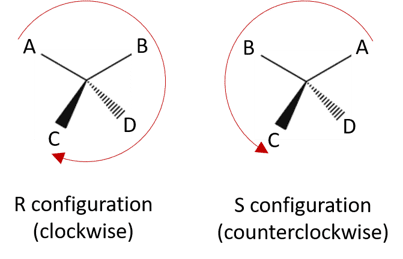
- The strategy to determine that chiral center has R or S configuration is based on symmetry properties of tetrahedral carbon atom.
- Firstly assign priorities to the groups attached to chiral center.
- Orient the molecule so the group of lowest priority points directly away from your eye.
- Follow the direction of remaining groups from the group of highest priority to the group of lowest priority.If procession is in clockwise direction then configuration is designated as R configuration.If procession is counterclockwise then the configuration is designated as S configuration.
How to assign priorities to the groups attached with chiral center?
To assign priorities to groups attached to chiral center following rules are followed:
- We assign priority to groups based on atomic number of atoms attached directly to chiral center.Higher atomic number atoms get higher priorities.
For example:
1-bromo-1-fluroethane, the order of priorities is Br>F>C>H on the basis of their atomic numbers as 35>9>6>1.
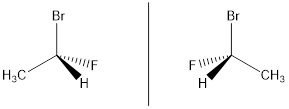
2. When priorities are not assigned on the basis of the atomic number of atoms attached directly to chiral center.Thn proceeds away these atoms from chiral center and examine the next sets of atoms for the differences in atomic numbers of attached atoms.
For example:
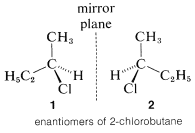
2-chlorobutanol, in these two carbon atoms, is attached to the chiral center. To identify which carbon atom has a higher priority. Note that the next atoms are H, H, H for the methyl group and H, H, C for the ethyl group. Thus ethyl group has a higher priority because of the greater atomic number of a carbon atom.

Enantiomers of 2-chlorobutane are as follow:
3. Groups containing multiple bonds are assigned priority if the both atoms are double or triple bonded.If vinyl-group is equivalent to 2-butyl group these are called phanton atoms.Phanton atoms don’t include the hydrogen atoms to complete valenceis.
For example:
In given below compound each carbon of 1 is the mirror image configuration of carbons in 4.
- The C-2 of 1 is R and C-2 of 4 is S.
- The C-3 of 1 is S and C-3 of 4 is R.
- Molecules themselves are mirror images but are non-superimposable.
- They are enantiomers.

Diastereomers:
The second type of stereisomers is diastereomers that are non-superimposable ,non mirror images are called diastereomers.Diastereomers have same molecular formula and sequence of bonded elements having different spatial arrangement of groups and are non-superimposable or non mirror images.Epimers are diastereomers.
The given diagram helps to understand diatereomers.

Mesoisomers:
The third type of stereisomers is meso-isomers. This appears when a molecule with several stereogenic center and has internal plane of symmetry. This can happens when two of stereogenic centers are attached to same four different valences.
Compounds that have chiral centers but has a plane of symmetry and superimposable are called meso-isomers.
For example:
2,4-dibromopentane has two stereogenic centers and has four stereoisomers,5-8.In which 6 and 7 are enantiomers and 5 and 6 are diastereomers so on.But in real three stereoisomers are present. A pair of enantiomers and a mes-isomer that is diastereomeric with enantiomeric pair.
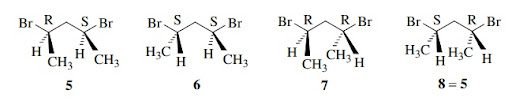
In the above example 5 and 8 are identical.There are two chiral centers in 5 and 8.And the molecule is achiral because it has an internal mirror plane.It has internal plane of symmetry.Structure 8 is superimposable on 5 by 180° of rotation.Thus it is same compound and these molecules are meso-isomers.
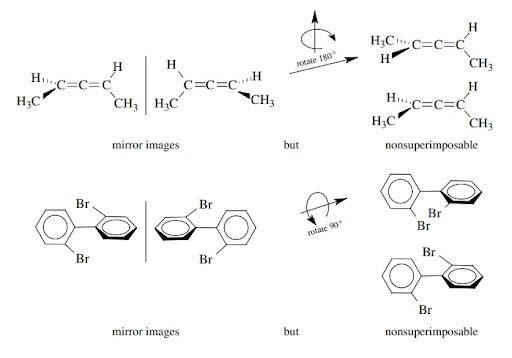
Molecules having no chiral center but are chiral:
There is another type of molecules that are chiral but they don’t have a chiral center. So molecular chirality arises due to presence of a screw axis in the molecule.
For example:
Alkenes and Biphenyls are common example of this type of compounds that are chiral but don’t have any chiral center. They exist as enantiomers.
Molecules having more than one stereocenter:
The molecules that have more than one stereocenter there is possibility that they are both enantiomers and diastereomers. To differentiate between diastereomers and enantiomers each of stereocenter is specified or identified.
For example:
If we take the example of aldotetroses that has two stereocenters and has four stereoisomers as given below in the figure.
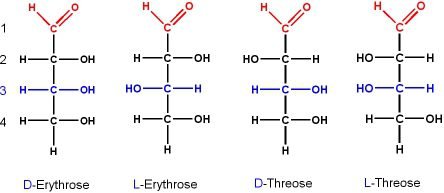
Figure show the four stereoisomers of aldosteroses. Then enantiomeric relation between D and L- threose is specified or identified by 2S, 3R and 2R, 3S configurations. And the enantiomeric relation between D and L -ethyrose is specified by 2R, 3R and 2S, 3S configurations. Since threose and ethyrose are diastereomers.
On the other hand the diastereomeric relation is obvious without specifying the configuration as the different spatial arrangement of -OH groups extending from chain in Fisher-projections. These are non -superimposable and mirror images.
Conclusion:
In this content we discuss all about chirality the stereochemical feature of tetrahedrally bonded compounds. How we configure or identify a chiral center? And also discuss about the compounds having chiral center or not what they have have configuration. And the examples proves that presence of chiral center in a molecule leads to stereoisomers.



Leave a Reply