
What is a carboxylic acid?
The compounds having hydroxyl group attached with carbonyl carbon have acidic nature are called carboxylic acid. The acidity of carboxylic acid is based on the resonance stability structures and the removal of H ion.
general formula: RCOOH
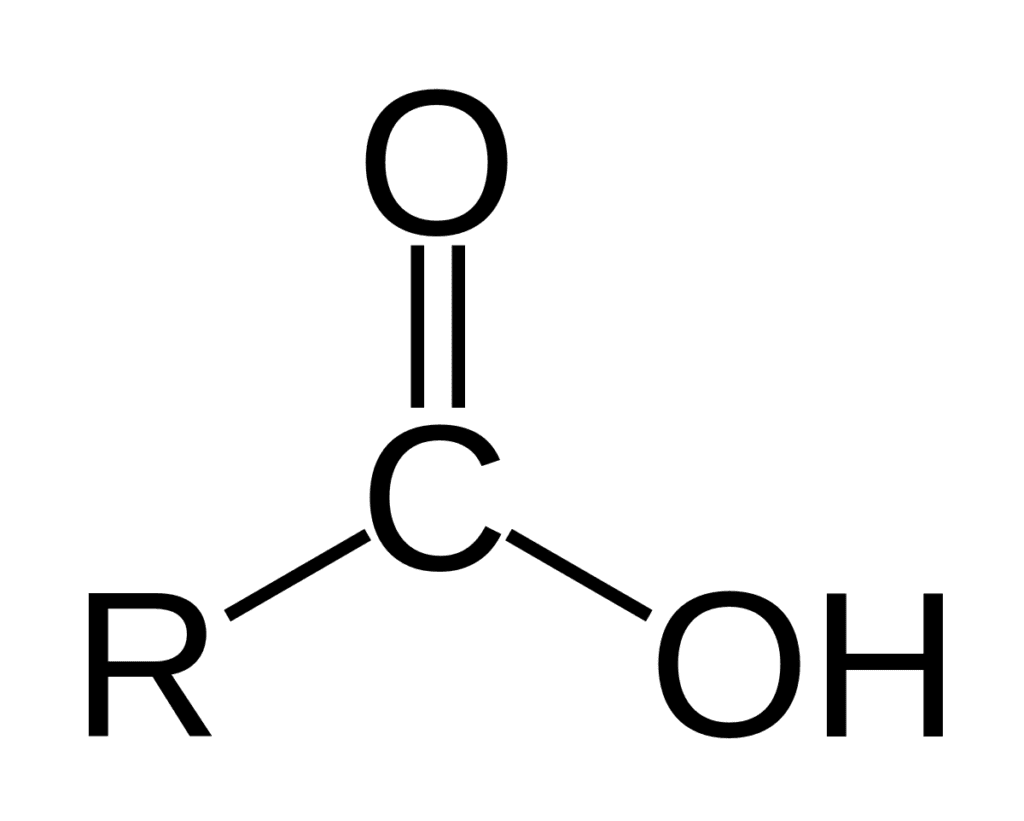
R refers to the hydrogen, aliphatic or aromatic group. carboxylic acid occurs widely in nature includes amino acids and fatty acids. formic acids are the simplest carboxylic acid.
Carboxyl is a weak acid / Acidity of Carboxylic acid:
The carboxyl group is the functional group in the carboxylic acids involved in most carboxylic acid reactions. Carboxyl is weak acid cause they are partially ionized in a solution. In the COOH carboxyl group, the hydrogen gives its electron to the oxygen which appears as a negative charge on it and itself attaches with the water molecule resulting in the formation of hydronium ion. As the carboxyl group releases a proton (hydrogen) in the solution, it’s an acid. But half of the carboxyl group ionize in the solution which makes it a weak acid.
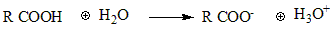
Ionization constant Ka
To describe the extent to which carboxyl is ionized, an ionization constant is used which is called as acidity constant. It is represented by Ka.
The expression of Ka is:
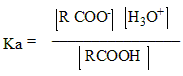
The concentration of water remains constant for most dilute acid, that is why it is not included in the expression. So that, the relative strength of acids is described by their Ka values. However, smaller the Ka value weaker the acid. The negative logarithm of Ka is pKa. pKa is used more often to express the acid strength. Weaker acid has a larger pKa value.
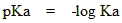
The pKa values of a carboxylic acid without any substitute range from 4 to 5.
Calculation of Carboxyl pKa:
Acetic acid is one of the common and simple carboxylic acids. In this, we will calculate the Ka and pKa values of acetic acid. Carboxylic acid‘s stability is also effected by the inductive effect.
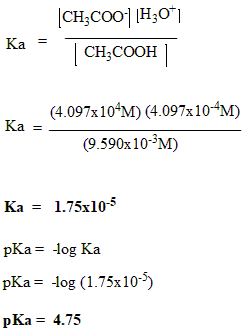
`
Resonance stability of carboxylate ion:
The acid strength of carboxylic acid is more than alcohol due to the resonance stability of its conjugate base. The resonance effect of carboxylate ion stabilizes it more than the carboxylic acid and increases its acidity. The resonance hybrid of a carboxylic acid is two nonequivalent structures that involve the separation of charges. But the resonance hybrid of carboxylate ion contains two equivalent structures with no separated charges. in resonance theory, structures with separated charges are less stable than the structures with the separated charges.
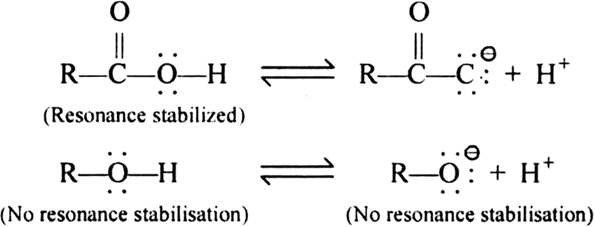
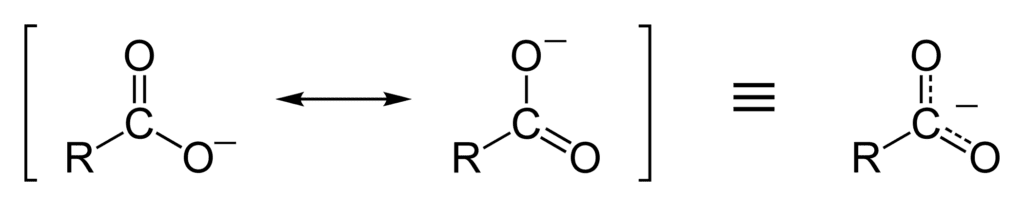
Decrease in acidity:
Within the carboxyl family, the acidity decreases with the increase in carbon atoms. the pKa value increases gradually as we move down the table of carboxyl acids with an increasing number of carbon atoms.


Leave a Reply