
How to convert alkene to alcohol? Alcohol can be prepared by the hydration of alkenes. The synthesis by converting alkene to alcohol is a commonly used method. The method to convert alkene into alcohol is described below:
Conversion of alkene to alcohol by hydration:

The process of the addition of water to an alkene is called hydration.
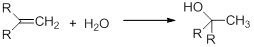
This reaction includes the process of pi bond breaking in the alkene and a hydroxyl bond in water. And the reaction includes C-H bond and C-OH bond formation. The reaction is usually exothermic by 10 – 15 kcal/mol.
Conversion of alkene to alcohol by acid-catalyzed hydration of alkene:
The addition of H2O to an alkene is quite slow. Therefore, the addition of acid is catalyzed by Lewis or Bronsted acids.

Mechanism of hydration in the conversion of alkene to alcohol:
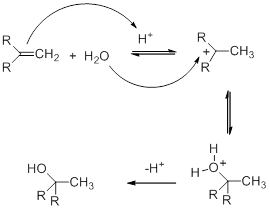
The hydration mechanism involves the electrophilic addition of an acid to doubly bonded carbons. This mechanism of hydration is completed in three steps.
Step 1
In this step, the alkene goes through protonation. protonation occurs by the attack of H3O+. As a result of this, a carbocation intermediate is formed.
Step 2
As nucleophile water attacks the carbocation. As a result oxonium ion is formed.
Step 3
By deprotonation, alcohol is formed. In the deprotonation of oxonium ion, a base is used which is either a conjugate base of the acid used as a catalyst.


Leave a Reply