Ionic Charge of S
Here we discussed the two methods to find out the ionic charge of S. The first method to find the sulfur charge is by using the periodic table. First of all, you know about the position of sulfur in the modern periodic table. Sulfur is located in group 3 and in period 16 or 6A just below the oxygen. Period 16 is also called the oxygen family because it is the first element of this period. All the elements present in the oxygen family have the same chemical properties. The charge of O is -2 so all the elements present in the oxygen family have a -2 charge.
The second method to find the charge is electronic configuration. If we look at the electronic configuration of sulfur:
1s2, 2s2, 2p6, 3s2, 3p4
The valence shell of the S atom is 3s2, 3p4. There are 6 electrons present in the outermost shell. It needs only two electrons to complete its octet. so it has a -2 charge.
What is the Oxidation State of S in H2SO4?
- The oxidation state of H is +1.
- The oxidation state of O is -2.
Let the oxidation state of sulfur is x,
2 (Oxdation state of H) + (Oxdation state of S) + 4 (Oxdation state of O) = 0
2 ( 1 ) + ( x ) + 4 ( -2 ) = 0
2 + x – 8 = 0
x = +6
The O.S of sulfur in sulfuric acid is +6.
Formal Charge of S in SO3
How to find the formal charge? SO3 exists in liquid form at room temperature which strongly fumes in the air. Sulfuric acid is formed when SO3 is combined with water. For gases, it acts as a drying agent. It acts as a solvent for the manufacture of H2SO4.
SO3 lewis structure, first, we calculate Q.
Q = Valance electron of all-atom + no of -ve charge – no of +ve charge
Q = (24 + 0 – 0)
Q = 24
B.P e– = 2 × no of bonds
B.P e– = 2 × 3
B.P e– = 6 e–
L.P e– = Q – B.P e–
L.P e– = 24 – 6
L.P e– = 18
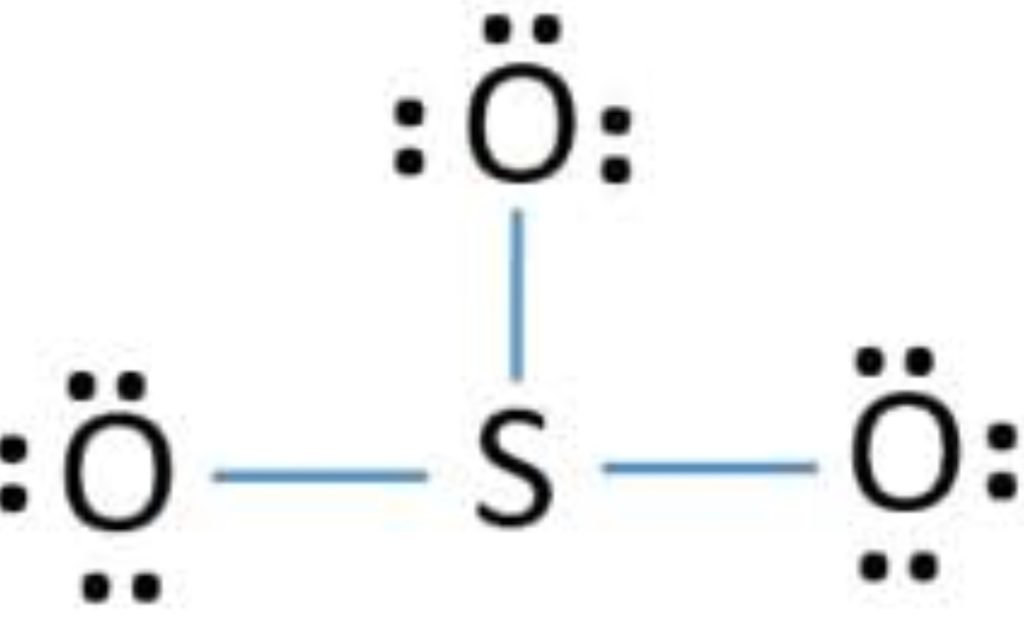
The sulfur has an incomplete octet so, the lone pair of one oxygen is shared with sulfur and makes a double bond.
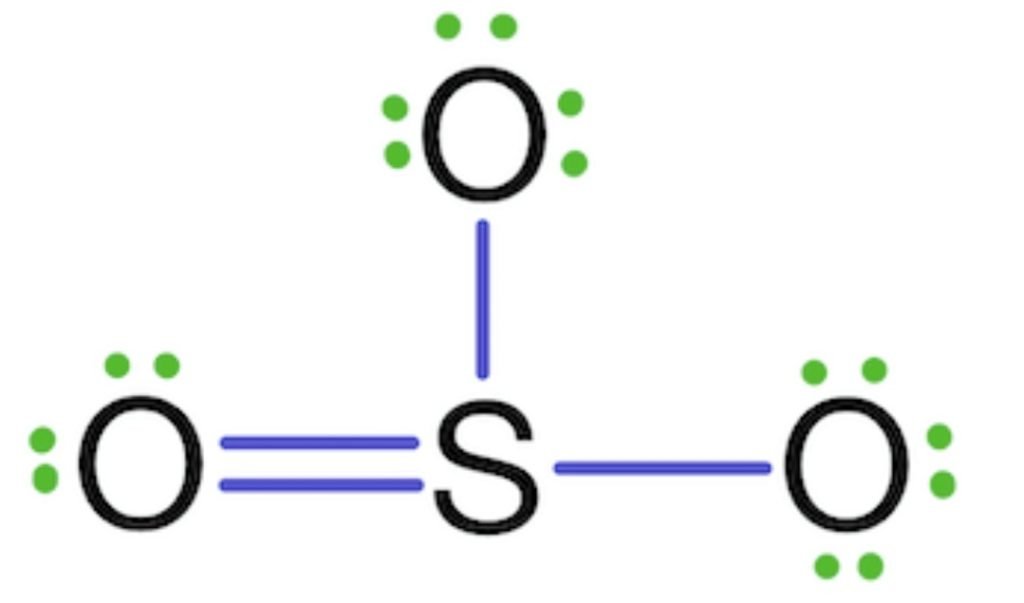
The octet of sulfur is complete but sulfur shows 4 co-valency rather than 6. So, the two lone pairs of two oxygens are shared with sulfur and make double bonds.
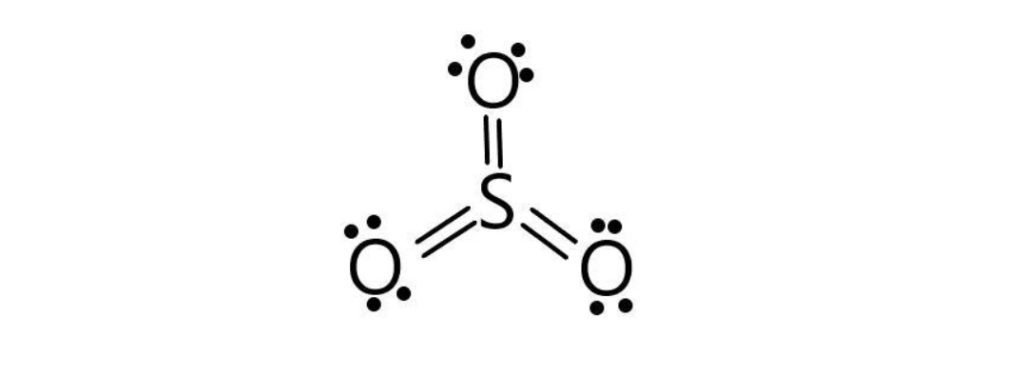
Formal Charge
F.C = Valance electrons – lone pair electrons – bond pair electrons/2
F.C(O1) = 6 – 4 – 4/2 = 6 – 4 – 2 = 0
F.C(O2) = 6 – 4 – 4/2 = 6 – 4 – 2 = 0
F.C(O3) = 6 – 6 – 4/2 = 6 – 6 – 2 = 0
F.C(S) = 6 – 0 – 12/2 = 6 – 0 – 6 = 0
SO3 has no formal charge.



Leave a Reply