
Born Haber Cycle-To Find Lattice Energy
Born Haber cycle is the special application of Hess’s law which is used to determine the lattice energy of binary ionic compounds. In the Born Haber cycle, there are many terms of energy is involved during the process.
The heat of Formation
“The amount of heat released when 1 mole of a compound is formed from its elements in the pure and stable state”
Na(s) + 1/2 Cl2(g) → NaCl(s) ∆Hf=?
The difference in lattice energy and heat of formation is that in lattice energy gaseous cation and anion combine and form 1 mole of an ionic compound but in heat of formation pure and stable state of an atom combines and forms an ionic compound.
Relation between ∆Hf and Lattice Energy
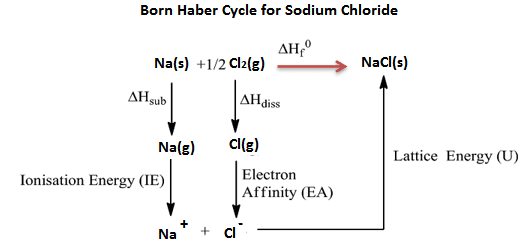
- In the formation of NaCl, two methods are used. One is the direct method (single step) and the other is an indirect method (several steps).
- The indirect method used to find the lattice energy of NaCl (ionic bond) is the Born Haber cycle.
- The energy released in the single step is called ∆Hf
- The energy released in several steps is called Lattice energy.
- The principle of the Born Haber cycle is Hess’s law (energy involve in single-step = energy involved in different steps)
∆Hf = ∆Hsub + I.E + ∆Hdiss + E.A + U
Now we calculate the lattice energy of sodium chloride,
In Single Step
Na(s) + 1/2 Cl2(g) → NaCl(s) ∆Hf = -411 KJ/mol
In Several Steps
Na(s) → Na(g) ∆Hsub = +108 KJ/mol
Na(g) → Na+(g) I.E = +496 KJ/mol
1/2 Cl2(g) → Cl(g) ∆Hdiss = +122 KJ/mol
Cl(g) → Cl–(g) E.A = -349 KJ/mol
Na+(g) + Cl–(g) → NaCl(s) L.E = ?
∆Hf = ∆Hsub + I.E + ∆Hdiss + E.A + U
-411 = +108 + 496 + 122 – 349 + U
U = = -411 -108 -496 -122 +349
U = – 788 KJ/mol
The lattice energy U is -788 KJ/mol
In the case of MgO,

The first electron affinity during the formation of O– is negative because it is exothermic and heat is evolved. But second electron affinity is positive because the second electron is repelled by an already present electron that’s why the energy is given by the adding electron. So, the second electron affinity is endothermic and positive.
∆Hf = ∆Hsub + I.E1 + I.E2 + ∆Hdiss + E.A1 + E.A2 + U
Lattice Energy Calculated by Born Haber Cycle For Some Ionic Compounds
| Compounds | Lattice Energy (KJ/mol) |
|---|---|
| LiF | 1030 |
| LiCl | 834 |
| LiI | 730 |
| NaF | 910 |
| NaCl | 788 |
| NaBr | 732 |
| NaI | 682 |
| KF | 808 |
| KCl | 701 |
| KBr | 671 |
| CsCl | 657 |
| CsI | 600 |
| MgCl2 | 2526 |
| SrCl2 | 2127 |
| MgO | 3795 |
| CaO | 3414 |
| SrO | 3217 |
| ScN | 7547 |
Factor On Which Lattice Energy Depends
Na+(g) + Cl–(g) → NaCl(s) (L.E)
- Energy is released due to attraction between Na+ and Cl–
- More the attraction = more the bond strength
- More the energy released = more the stability
F ∝ q1q2/ r2
F = K q1q2/ r2
- q1 and q2 are charged on ions.
- r is the distance between them.
F ∝ Lattice energy
Factor 1
Lattice Energy ∝ Z+Z–
Lattice energy is directly proportional to the charge on cation and anion.
Factor 2
Lattice energy ∝ 1/size
Lattice energy is inversely proportional to the size of the cation and anion.
Which one has the highest Lattice energy?
a) NaF
b) MgF2
c) AlF3
- Anion is the same in given compounds.
- Cation is different so, we see only the charge factor not the size factor because from left to right size is slightly increased. The charge on Na, Mg, and Al is +1, +2, and +3 respectively. The greater the charge, the greater the lattice energy.
Order
NaF < MgF2 < AlF3
Which one has the highest Lattice energy?
a) Na2O
b) MgO
c) Al2O3
- Anion is the same in the given compounds.
- Cation is different so, we see only the charge factor, not the size factor. The charge on Na, Mg, and Al is +1, +2, and +3 respectively.
Order
Na2O < MgO < Al2O3
Which one has the highest Lattice energy?
a) Li2O
b) Li3N
- Cation is the same in given compounds.
- Anion is different so, we see only the charge factor, not the size because nitrogen and oxygen are very close to each other. The charge on O and N is -2 and -3 respectively.
Order
Li2O < Li3N
Which one has the highest Lattice energy?
a) NaCl
b) KCl
- Anion is the same in given compounds.
- Cation is different so, we see only the charge size factor not the charge because both the Na and K have a +1 charge. The size of K is larger than Na. (greater the size, smaller the lattice energy)
Order
NaCl > KCl
Which one has the highest Lattice energy?
a) NaF
b) NaCl
c) NaBr
- Cation is the same in given compounds.
- Anion is different so, we see only the size factor not the charge factor because fluorine, chlorine, and bromine have a -1 charge. As we move from down the group in the periodic table size increases.
Order
NaF > NaCl > NaBr
Which one has the highest Lattice energy?
a) NaF
b) MgCl2
- Both the cation and anion are different.
- Compare the cation Na+ < Mg2+ (charge factor)
- Compare the anion F– < Cl– (size factor)
if both the size and charge factor fight with each other then the charge factor will be dominant over the size.
Order
NaF < MgCl2



Leave a Reply