
Here we discussed the enthalpy of the reaction, enthalpy of formation, and enthalpy of combustion. The heat of reaction is evolved or absorbed during the chemical reaction.
In a chemical reaction, old bonds of reactants are broken and new bonds of products are formed simultaneously. The heat is required to break the bonds of the reactants so this process is endothermic and heat is released during the bond formation so it is exothermic in nature. Let us a general chemical reaction:
Reactants → Products
∆H = HP – HR
- ∆H = Change in heat or enthalpy change
- HP = Heat content of the product
- HR = Heat content of the reactant
∆H may be positive or negative. In an exothermic reaction, energy is released so ∆H is negative. On the other hand, in an endothermic reaction, energy is absorbed so ∆H is positive.
∆H → negative → energy released → exothermic → HR>HP
∆H → positive → energy absorbed → endothermic → HR<HP
The specific heat capacity, activation energy, and temperature increase are factors that affect the heat flow within a system.
Graph of Heat of Reaction
Graph of Exothermic and Endothermic Reaction

Let us a general example to understand the concept of endothermic and exothermic reactions:
A + B → C + D + “x” Kcal ∆H = -“x” Kcal exothermic
A + B → C + D – “x” Kcal ∆H = +”x” Kcal endothermic
we can also write as
A + B + “x” Kcal → C + D ∆H = +”x” Kcal endothermic
Factors on which ∆H depends
1) The physical state of reactants and products
H2 (g) + 1/2 O2 (g) → H2O (g) ∆H1
H2 (g) + 1/2 O2 (g) → H2O (l) ∆H2
Where ∆H1≠ ∆H2
2) Allotropic form of reactants and products
C(diamond) + O2 → CO2 (g) ∆H1
C(amorphous) + O2 → CO2 (g) ∆H2
Where ∆H1≠ ∆H2
3) Solution in which reaction takes place (solvent)
4) Temperature and pressure (physical conditions)
Standard Heat of Reaction
The condition for standard enthalpy of reaction is at 298 K temperature, 1 atm pressure, and the concentration of an aqueous solution is 1 molar (1 mol/L). It is represented as ∆H°.
Let the standard enthalpy of some naturally occurring state substances be zero.
- ∆H°Cl2 = 0
- ∆H°O2 = 0
- ∆H°H2 = 0
- ∆H°Br2 = 0
- ∆H°N2 = 0
- ∆H°C (graphite) = 0
Carbon in graphite form is naturally occurring so the standard enthalpy of carbon in graphite form is zero but in diamond, the ∆H° is not zero.
- ∆H°S8 (orthorhombic) = 0
- ∆H°P4 (white) = 0
Enthalpy of Formation
The amount of heat absorbed or released when one mole of a compound is formed by its constituent elements in their free natural state.
H2 + 1/2 O2 → H2O (g) ∆H = -241.82 kJ/mol
The enthalpy of the formation of water is the enthalpy of water. Let the reaction take place at standard state. (1 atm, 25°C)
∆H = HP – HR
∆H°f = HH2O – (HH2 + HO2)
∆H°f = HH2O – (0)
∆H°f = HH2O
So enthalpy of any component is the enthalpy of the formation of that compound.
In the formation of CO2, at 1 atm and 25°C, when one mole of carbon reacts with one mole of oxygen to form one mole of carbon dioxide. The heat of the formation of CO2 is -393.5 kJ/mol.
C(s) + O2 → CO2 ∆H = -393.5 kJ/mol
∆H = HP – HR
∆H°f = HCO2 – (HC + HO2)
∆H°f = HCO2 – (0)
∆H°f = HCO2
The enthalpy of formation of carbon dioxide is the enthalpy of carbon dioxide.
When 1 mole of H2 reacts with one mole of Cl2, 2 moles of HCl are produced. The ∆H for the reaction is -44 Kcal.
H2 + Cl2 → 2HCl ∆H = -44 kcal
Enthalpy is an extensive property. and heat of formation is defined for only one mole.
∆H°f (HCl) = -22 kcal
∆H = HP – HR
-44 = 2HHCl – (0)
-22 kcal = HHCl
The enthalpy of HCl is the enthalpy of formation of HCl.
Numericals
Calculate enthalpy change (∆H) for the given reaction:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)
The enthalpy of formation of CH4 is -74.5 KJ/mol, CO2 is -393.5 kJ/mol, and H2O is -285.8 KJ/mol.
∆H = HP – HR
∆H = (HCO2 + 2HH2O) – (HCH4 + 2HO2)
∆H = (-393.5 + 2×-285.5) – (-74.5 + 2×0)
∆H = -964.5 + 74.5
∆H = -890 KJ/mol
Calculate ∆H and∆U for the given reaction:
NH4NO3(s)⟶ N2O(g)+2H2O(l)
The heat of formation of NH4NO3(s), N2O, and H2O are −367.5 kJ, +81.46 kJ, and −285.78 kJ respectively at 1 atm and 25°C.
∆H = HP – HR
∆H = (HN2O + 2HH2O) – (HNH4NO3)
∆H = (+81.46 + 2×-285.5) – (-367.5)
∆H = -490.1 + 367.5
∆H = -122.6 KJ/mol
As
ΔH=ΔU + ΔngRT
ΔU=ΔH − ΔngRT
Δng = np-nR
Δng = 1 – 0 = 1
ΔU=-122.6 − (1) (8.314) (298) / 1000
ΔU=-122.6 − 2.47
ΔU= -125.07 KJ/mol
Enthalpy of Combustion
The amount of heat released when 1 mole of a compound is combusted completely in excess of air or oxygen. It is represented by ΔHc. ΔHc is always negative or exothermic in nature.
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ΔHc = -891 KJ/mol
The calorific value gives an idea about which fuel is better in terms of energy.
Experimental Methods for Measuring Heat of Reaction
In chemistry, it’s important to measure the heat of reaction correctly to understand energy changes in chemical reactions. Different methods have been made to find the heat of reaction, each with its own pros and cons.
Calorimetry Measurements
Calorimetry measures heat changes in chemical reactions. Scientists use this to find out how much heat is released or absorbed. It helps them understand reactions better.
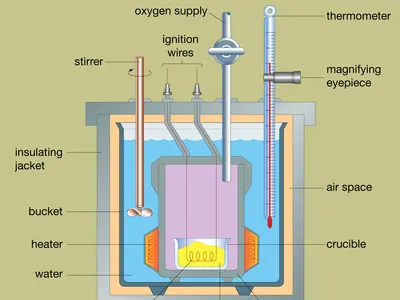
Bomb Calorimeters for Combustion Reactions
When studying combustion reactions, bomb calorimeters are commonly employed. These specialized devices are designed to contain the reactants within a sealed vessel known as a bomb.
The reactants undergo combustion, releasing heat energy that is transferred to the surrounding water in the calorimeter. The temperature change in the water is then measured, allowing for the calculation of the heat released by the reaction.
Solution Calorimeters for Aqueous Reactions
For reactions in water, solution calorimeters are better. They have two compartments: one with water and one with the reactants dissolved in a solvent. When these solutions are mixed, a chemical reaction happens and either heat is absorbed or released.
Scientists use a thermometer to measure temperature changes in the water.
This helps them figure out if heat was gained or lost during the reaction. By controlling and monitoring factors like initial temperatures and masses of reactants and solvents, they can get accurate measurements.
Importance of Accurate Heat Measurements
Accurate measurement of heat changes is crucial because it allows scientists to determine important thermodynamic quantities such as enthalpy (ΔH) and entropy (ΔS). These values provide insights into the energy changes and disorders associated with a reaction.
By understanding these thermodynamic properties, scientists can predict the feasibility and spontaneity of reactions under different conditions.
Furthermore, accurate heat measurements enable researchers to compare the efficiency of different reactions or processes.
This information is valuable in industries such as chemical engineering, where optimizing reaction conditions is essential for maximizing productivity and minimizing costs.
Summing Up
The heat of reaction is an important concept in chemistry. It helps scientists understand and predict how chemical reactions behave. It tells us if a reaction gives off heat (exothermic) or absorbs heat (endothermic).
By calculating the heat of reaction, scientists can learn more about the thermodynamics and make smart choices in chemical processes.
To delve further into this topic, it is essential to explore the experimental methods used to measure heat of reaction accurately.
Understanding its applications in chemical processes can provide valuable insights for industries such as pharmaceuticals, energy production, and materials science.
By considering these aspects, readers will be able to appreciate the importance of heat of reaction in various scientific fields and apply this knowledge effectively.


