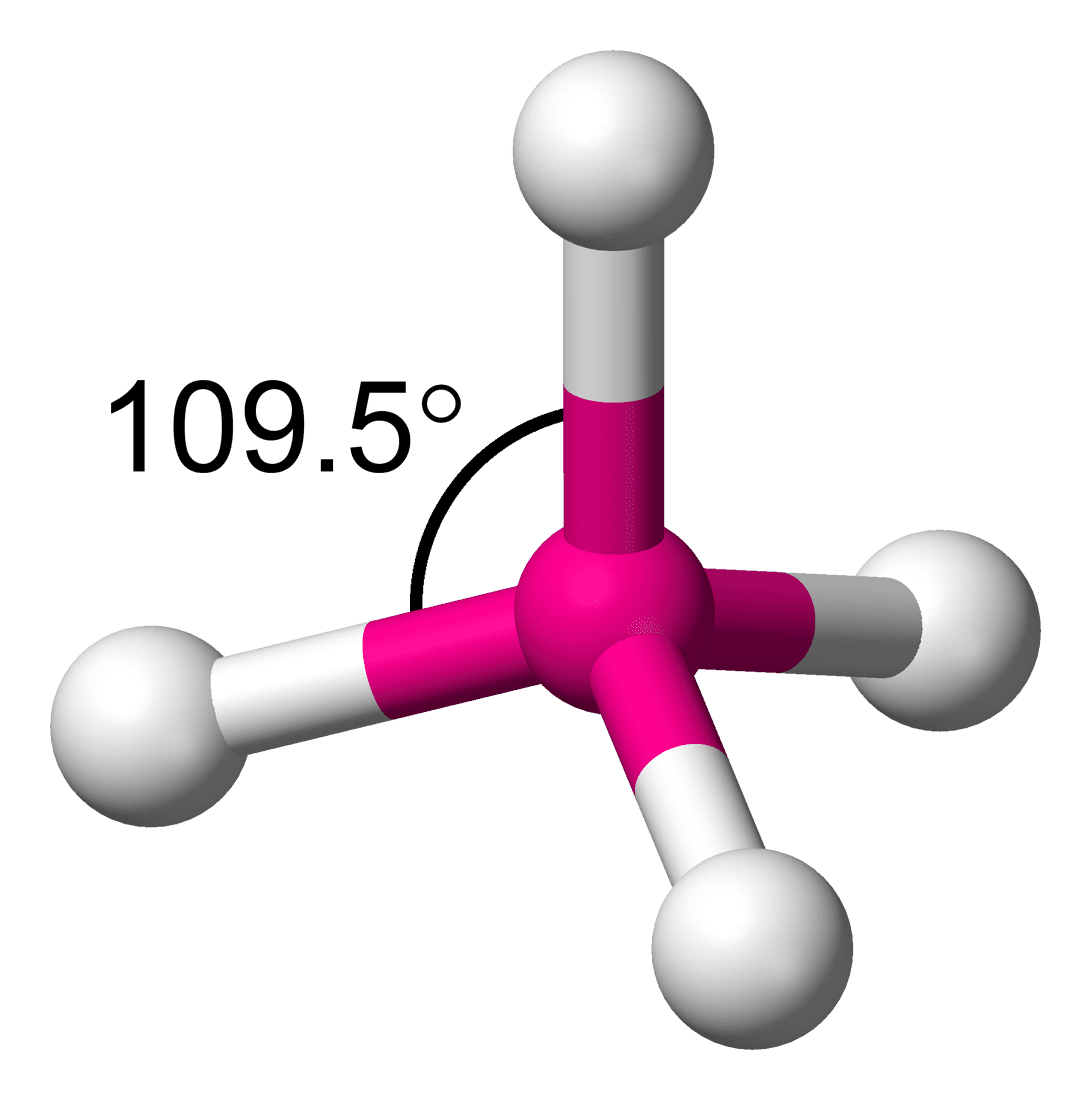
In a molecule, the angle between two bonds is called the bond angle. It is not important to know the bond angle, but how determining the bond angle is important. If we have two molecules NH3 and PH3, which one has more bond angle? To compare the bond angle between two molecules, it is important to understand the steps to find the bond angle.
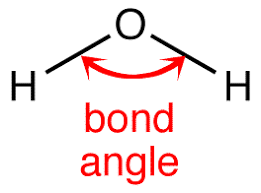
What are the steps to determine the Bond Angle?
- To check the hybridization of the central atom
- If the hybridization of the central atom is the same, then check the lone pair on the central atom
- If the hybridization of the central atom and the lone pair on the central atom is the same, then check the electronegativity of the central atom and surrounding atoms depending on the situation that we discussed in detail.
To Check the Hybridization of Central atom
First, check the hybridization of the central atom. The formula to calculate the hybridization of the central atom is:
Z = 1/2 (Number of valence electrons of central atom + number of negative charge – number of +ve charge + number of mono-valent atoms)
- If the hybridization is sp3, the geometry of the molecule is tetrahedral and the bond angle is 109.28°.
- If the hybridization is sp2, the geometry of the molecule is trigonal and the bond angle is 120°.
- If the hybridization is sp, the geometry of the molecule is linear and the bond angle is 180°.
So the order will be
sp3 < sp2 < sp
If the Hybridization of the Central Atom is the Same, then Check the Lone pair on the Central Atom
“More the lone pair on the central atom, the lesser will be the bond angle”
If the Hybridization of the Central Atom and the Lone pair on the Central Atom is the Same, then
- If the central angle is different and the surrounding atoms are the same, check the electronegativity of the central atom. The more the electronegativity of the central atom, the greater will be the bond angle.
- If the central angle is the same and the surrounding atoms are different, check the electronegativity of the surrounding atom. The more the electronegativity of the surrounding atom, the lesser will be the bond angle.
Practice Question
Which has more the bond angle BeCl2, BCl3, or CCl4?
First, check the hybridization (Z) for all molecules.
Hybridization of BeCl2
Z = 1/2 (Number of valence electrons of central atom + number of negative charge – number of +ve charge + number of mono-valent atoms)
Z = 1/2 (2 + 0 – 0 + 2)
Z = 1/2 (4)
Z = 2
sp hybridization
Hybridization of BCl3
Z = 1/2 (3 + 0 – 0 + 3)
Z = 1/2 (6)
Z = 3
sp2 hybridization
Hybridization of CCl4
Z = 1/2 (4 + 0 – 0 + 4)
Z = 1/2 (8)
Z = 4
sp3 hybridization
As we know that the bond angle of sp, sp2, and sp3 is 109.28°, 120°, and 180° respectively. So the order will be
BeCl2 > BCl3 > CCl4
Compare the bond angle in NO2+ and NO2−?
Check the hybridization for both NO2+ and NO2−.
Hybridization of NO2+
Z = 1/2 (5 + 0 – 1 + 0)
Z = 1/2 (4)
Z = 2
sp hybridization (Bond angle = 180°)
Hybridization of NO2−
Z = 1/2 (5 + 1 – 0 + 0)
Z = 1/2 (6)
Z = 3
sp2 hybridization (Bond angle = 120°)
The order of bond angle is NO2+ > NO2−.
Compare the bond angle in CH4, NH3, and H2O?
Calculate the hybridization of CH4, NH3, and H2O.
Hybridization of CH4
Z = 1/2 (4 + 0 – 0 + 4)
Z = 1/2 (8)
Z = 4
sp3 hybridization
Hybridization of NH3
Z = 1/2 (5 + 0 – 0 + 3)
Z = 1/2 (8)
Z = 4
sp3 hybridization
Hybridization of H2O
Z = 1/2 (6 + 0 – 0 + 2)
Z = 1/2 (8)
Z = 4
sp3 hybridization
We observe that the hybridization of CH4, NH3, and H2O is the same, so the hybridization is not enough to determine the bond angle. Now move to the next step and check the lone pair on the central angle. How to find the lone pair?
Lone pair on the central angle = Z – number of surrounding atoms
Lone pair on CH4
Lone pair = Z – no. of the surrounding atom
Lone pair = 4-4
Lone pair = 0
Lone pair on NH3
Lone pair = Z – no. of the surrounding atom
Lone pair = 4-3
Lone pair = 1
Lone pair on H2O
Lone pair = Z – no. of the surrounding atom
Lone pair = 4-2
Lone pair = 2
As we know that the more the lone pair on the central atom, the lesser the bond angle. So the order will be
CH4 > NH3 > H2O
Remember the repulsion order:
lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
Compare the bond angle in SO3 and SO2?
Check the Z value for both SO3 and SO2.
Hybridization of SO3
Z = 1/2 (6 + 0 – 0 + 0)
Z = 1/2 (6)
Z = 3
sp2 hybridization
Hybridization of SO2
Z = 1/2 (6 + 0 – 0 + 0)
Z = 1/2 (6)
Z = 3
sp2 hybridization
Hybridization is the same, now check the lone pair on the central atom.
- Lone pair on SO3: Lone pair = 3-3 = 0
- Lone pair on SO2: Lone pair = 3-2 = 1
The more the lone pair, the lesser will be the bond angle. So the order will be SO3 > SO2.
Compare the bond angle in NH3 and PH3?
Hybridization of NH3
Z = 1/2 (5 + 0 – 0 + 3)
Z = 1/2 (8)
Z = 4
sp3 hybridization
Hybridization of PH3
Z = 1/2 (5 + 0 – 0 + 3)
Z = 1/2 (8)
Z = 4
sp3 hybridization
Hybridization is the same, now check the lone pair on the central atom.
- Lone pair on NH3: Lone pair = 4-3 = 1
- Lone pair on PH3: Lone pair = 4-3 = 1
If the Z value and the lone pair on the central atom are the same, then move on to the next step to predict the bond angle. If the central angle is different and the surrounding atoms are the same then check the electronegativity of the central atom. The greater the electronegativity of the central atom, the greater will be the bond angle. The electronegativity of nitrogen is greater than phosphorus. So the order will be:
NH3 > PH3
Compare the bond angle in COF2 and COCl2?
Hybridization of COF2
Z = 1/2 (4 + 0 – 0 + 2)
Z = 1/2 (6)
Z = 3
sp2 hybridization
Hybridization of COCl2
Z = 1/2 (4 + 0 – 0 + 2)
Z = 1/2 (6)
Z = 3
sp2 hybridization
- Lone pair on COF2: Lone pair = 3-3 = 0
- Lone pair on COCl: Lone pair = 3-3 = 0
If the central atom is the same and the surrounding atoms are different then check the electronegativity of the surrounding atoms. Thr greater the electronegativity of the surrounding atom, the lesser will be the bond angle. So the order will be COF2 < COCl2.



Leave a Reply