
The Bunsen burner is a device widely used in scientific laboratories to heat substances. It is used to sterilize small objects, heat chemicals, burn broken glass, and for many other purposes. A Bunsen burner is a small gas burner with an adjustable flame, where you can manipulate the amount of gas and air. The burner is named after Robert Wilhelm Bunsen, a German chemist credited with inventing the device, based on his assistants Michael Faraday and Peter Desaga.
Knowing the History of Bunsen Burner
Like many scientific inventions, the burner reflects the name of the scientist overseeing the laboratory where it was invented, not the name of the actual inventor.
Robert Wilhelm Bunsen was a well-known chemist in Germany in the mid-1800s who became particularly interested in examining the spectra of different elements, that is, the unique set of wavelengths, or colors, of light, that each emits when heated. . To do this effectively, he needed something that would produce a scorching flame with low brightness so that its light would not obscure the spectrum.
He came up with the idea of mixing natural gas with air before combustion and instructed a lab technician, Peter Desaga, to design and build the burner.
The resulting device allowed significant control over the height and intensity of the flame and was very successful. It quickly became associated with the Bunsen laboratory and was popularly known as a Bunsen burner.
Not long after its invention, the device allowed Bunsen to discover two new elements, cesium, and rubidium, from their never-before-seen spectra.
The elements are named after the spectral lines they produce, sky blue and dark red, respectively. Other chemists discovered several other new elements in this way.
How Bunsen burner is constructed??
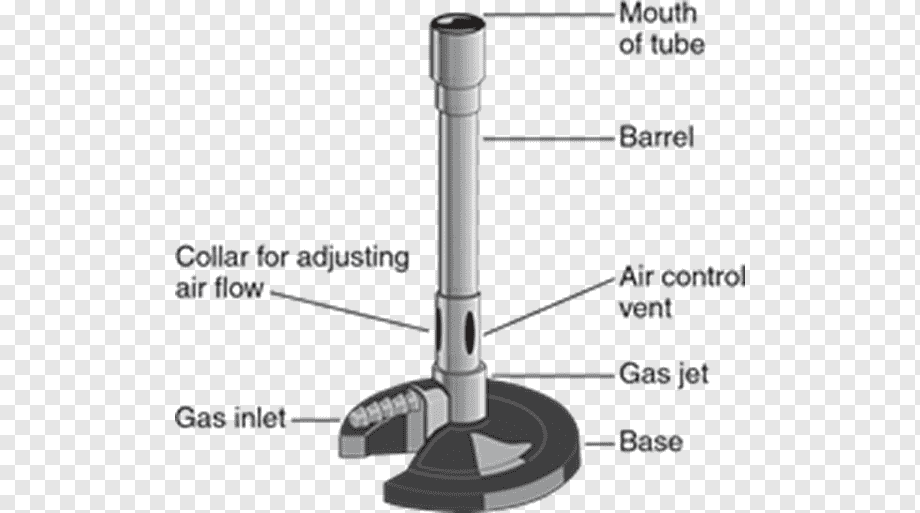
Observing the Bunsen burner more closely, it can be seen that it is made up of three parts: base, ring, and tube.
There is a fitting ring between the base and the tube with two holes or windows. In the tube, there are other windows, and air intake takes place through the paired windows. When they are juxtaposed, we say they are open; when the ring completely covers the tube window, we say they are closed.
The constitution of a Bunsen burner includes a vertical metal tube approximately 13 cm long, which is attached to a base. This base consists of a nozzle to connect with a fuel source and a gas valve, and a combustion regulator to control the amount of air through small holes in the base of the tube. The gas mixes with the air at the bottom of the tube and then travels to the top of the tube, where it can be lit with a match or lighter, producing a great, luminous flame, which can be controlled either in its height and intensity. Several types of Bunsen burners are available for use with liquefied petroleum gas (LPG), coal gas, coal, and natural gas.
Flame Production in Bunsen burner
The flame produced by the Bunsen burner varies in color (yellow-orange to blue) and temperature (300º C to 1600º C). When the air (oxygen) holes are fully closed at the bottom of the device, the gas will only mix with the ambient air after it has exited the tube at the top. This mixture produces a bright yellow flame known as the “Safety Flame” as it is easier to see and less hot. This flame is also called a “dirty” flame because it leaves a layer of carbon (soot) on top of heated heat. The temperature reached is around 300°C.
The type of flame most used for heating is the blue flame, which is also referred to as an invisible flame that is hardly seen in a well-lit room. This flame reaches a suitable temperature for heating. To produce this bluish flame, the opening of the air holes at the base of the Bunsen burner must be regulated so that the oxygen mixes with the gas, making it burn more efficiently.
What are the parts of flame?
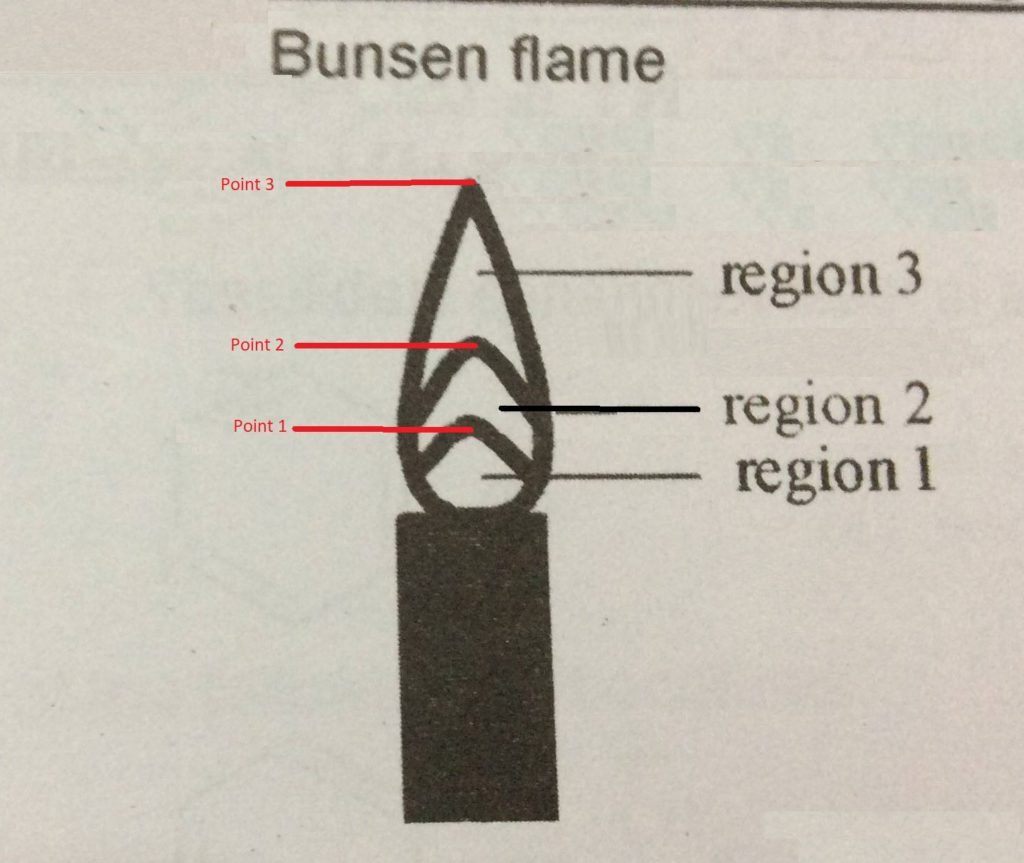
- Flame neutral zone: region close to the tube mouth; it does not burn the gas. It is considered cold compared to the other areas.
- Flame reducing zone (zone 1): it is above the neutral zone and forms a small “cone,” where the combustion of the gas begins. The temperature is much lower than that of the oxidizing area.
- Flame oxidizing zone (zone 2): comprises the entire region above and around the reducing site; in it, the combustion of the gas is complete. It is scalding: its temperature can reach 1100 °C.
How to observe this flame??
To observe the Bunsen burner flame.
- First, check that the ring windows are closed: The Bunsen burner must be lit with the windows closed to prevent the flame from collecting inside the tube.
- Control the amount of gas with the valve and gradually turn the ring until the Bunsen burner windows are entirely open. Note the changes in the flame (complete combustion).
- Pass another porcelain capsule over the flame.
To identify the flame regions:
- Go through zone 1 with the tip of a used matchstick, observing what happens. Write down. Repeat the experiment moving the match to zone 2. Observe and note.
- Place the toothpick horizontally to go through zones 1 and 2 simultaneously.
How Bunsen Burner works??
The gas mixes with the air at the bottom of the tube and rises to the top of the burner, where it can be lit with a match or lighter.
With the air holes closed, a smoky yellow flame is produced due to the incomplete combustion of the carbon. Natural gas consists primarily of methane, a carbon-hydrogen compound; if there is not enough air, not all the carbon burns, forming tiny soot particles that glow yellow in the heat. This flame is not used for heating as it deposits soot on anything inside or above it and, in any case, is not hot enough for many purposes.
When the holes are opened, the air is drawn into the burner, allowing complete combustion of the natural gas fuel, producing a blue flame. This flame is much hotter – reaching up to 1500°C – and is used for heating purposes. It usually has a light outer cone and a more intensely blue inner cone, the tip of which is the hottest part of the flame.
The device can be adapted to run on propane or butane from cylinders to be used in places without a gas supply.
The air hole allows the premixing of air and gas before combustion at the top of the chimney. A collar around the chimney base, with a hole that lines up with the air hole, acts as an air regulator, allowing the premix air to be adjusted.
Air is drawn into the air hole due to the Venturi effect.
A fluid flow transfers energy in three ways: potential energy, pressure, and kinetic energy. Bernoulli’s principle states the
A change in velocity must result in a change in potential energy or a change in fluid pressure due to the conservation of energy. When the velocity of a fluid flow increases, it is usually the pressure that decreases.
As the gas in a Bunsen burner flows through the chimney, it has a lower pressure than the stagnant air.
This pressure difference causes air to be pulled into the air hole as gas flows through it, a phenomenon known as the Venturi effect.
As the air hole is opened, the flame progresses from a choppy orange to a more stable orange, a steady purple, and a roaring blue flame.
Uses of Bunsen burner:
Following are the uses of the bunsen burner:
The primary use of the Bunsen burner is as a means of strongly heating substances during chemical experiments, and it is often used to heat the material in a glass test tube.
If extreme heating is required – strong enough to melt the glass – a small porcelain dish known as a crucible can be used.
Bunsen burners can also be employed in a crude chemical analysis called a flame test. Many elements, particularly metals, emit specific colors when heated in a flame. These elements can often be detected by placing them in a Bunsen flame; for example, sodium gives a yellow flame, and potassium gives lilac and barium green. This method has its limits and disadvantages – for example, the solid yellow color of sodium can mask the presence of other metals and has largely been replaced by spectroscopes. Still, it can be a quick functional test in some cases.
Safety measures for Bunsen Burner
Many laboratory accidents involve burns related to open flames, and as a result, new students are carefully trained in the use of gas burners. The student should always wear protective eyewear and make sure hair and clothing are secure not to contact the flame.
Flammable substances must be kept away from the heat source, and someone must be present at all times to supervise them.
When lit but not in use, the air hole must be closed to visible the flame: the hot blue flame can be difficult to see in intense light.
When heating small objects in the flame, a pair of tweezers should be used. Larger objects, such as flasks and beakers should be placed on a shelf or clamped with tweezers.
The flexible rubber hose connecting the Bunsen burner to the gas burner on the laboratory bench must be secure, with no evidence of leakage.
Care should be taken when touching objects that have been exposed to the heat of the burner, especially glass objects, which may remain hot for some time.


Leave a Reply