
This article is based on geometrical isomers & stereoisomerism. Two or more compounds that have the same molecular formula but have different structural formulas and different physical and chemical properties are called isomers. And the method of isomer formation is called isomerism. The difference in their properties is due to the different modes of arrangement of atoms within molecules. There are two main types of isomerism:
- Structural isomerism
- Stereoisomerism
| STRUCTURAL ISOMERISM | STEREOISOMERISM |
| Structural isomers have the same molecular formula but different structural formulas. The process by which structural isomers are formed is called structural isomerism. | Stereoisomers have the same molecular formula, same structural formula, and have different spatial arrangements of atoms/groups are called Stereoisomers |
| The structural isomers are distinguished by two-dimensional structures. They may be of five types: 1. Chain isomerism 2. Position isomerism 3. Functional isomerism 4. Metamerism 5. Tautomerism | Stereoisomers cannot be distinguished by two-dimensional structures but only by three-dimensional structures. They are roughly divided into two types: 1. Configurational isomerism 2. Conformational isomerism |
Stereoisomerism and its types:
Stereoisomers that are interconverted only by making and breaking the bond and are isolated are called Configurational isomers and the process, by which they separate is called configurational isomerism. The products or structures of these stereoisomers are called configurations. The configurational stereoisomerism is further divided into two types:
- Optical isomerism
- Geometric isomerism
- Enantiomers: These are the isomers that have mirror images. These show optical isomerism.
- Diastereomers: These are the isomers that are non-mirror images. These show both optical and geometric isomerism.
Stereoisomers that can be converted only by rotation about a single bond are called conformational isomers. And the process by which they separate is called conformational isomerism. The structures formed by conformational isomerism are called conformations or rotomers.

Geometrical isomerism/isomers:
Geometrical isomerism arises when atoms and groups are arranged differently in space due to the restricted rotation of bonds in molecules. Finding geometrical isomers should have the following formula. i.e. 2n where “n” is the number of the double bonds. No. of double bonds represents the no. of geometrical isomers.
For example, 2,4-heptadiene possesses two double bonds so by the formula it possesses 4 geometrical isomers. The following diagram represents the four isomers of 2,4-heptadiene.
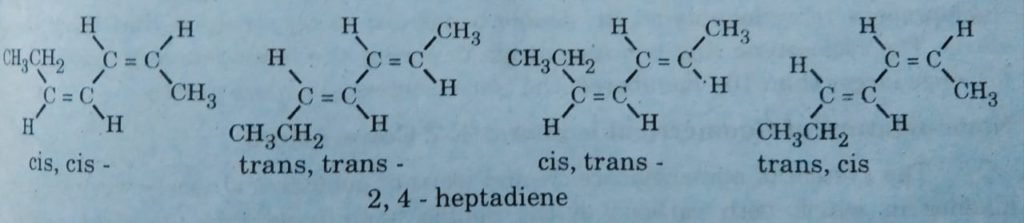
Cis-trans geometrical isomers:
Basically, cis-trans isomerism is also called geometrical isomerism. Cis-trans comes from the Latin word that means “to the side of” or “to another side” respectively. Cis-isomer is one in which two similar groups are on the same side of the double bond. Trans-isomers are the isomers that have similar groups on the opposite side to the double bond. There are some of the essential conditions for cis-trans isomerism:
- There should be a presence of a double bond in the compound which contains one sigma and one pie bond. Sigma bond is a single bond so it allows the free rotation which is unable to convert cis form to trans form. But the presence of a pie bond allows restricted rotation due to head-on-head overlapping.
- For cis-trans isomerism, there should be separate groups on the adjacent carbons. The same groups on the same carbon cannot permit a compound to show cis-trans isomerism.
- Geometrical isomerism occurs when there is a different electron density on the entire molecule.
If we imagine the structures 1,2-dichloroethene, cis-2-butene & trans-2-butene there is a double bond between the C=C. These both are sp2 hybridized and have a bond angle of 120 degrees and trigonal planar geometry. The C=C double bond contains one sigma and one pie bond which does not allow any kind of rotation. So the first condition is satisfied. The sigma bond is formed by the sp2 hybrid orbitals overlapping. The pie bond is formed by the overlap of the p-orbital. The maximum overlap between the p-orbital occurs when the p-orbitals are exactly parallel.
If we rotate a carbon of double bond at 90 degrees, the p-orbital become perpendicular to each other and there is no net overlap between them. As a result, the pie bond breaks, and only the sigma bond remains. Therefore, neither of the doubly bonded carbon atoms can be rotated about the double bond without breaking the pie bond. The strength of the pie bond is 263KJ/mol which is a barrier to rotation. Therefore, there is no rotation is between the C=C double bond.
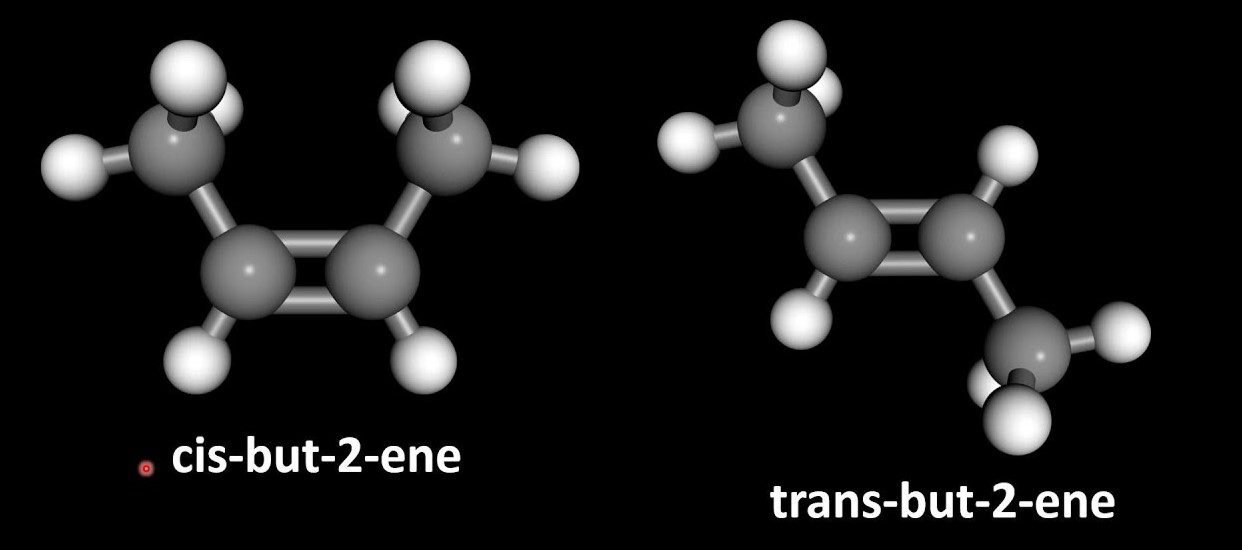
This diagram is showing both conditions of the geometrical isomerism. i.e. restricted rotation and groups on the opposite side of the adjacent carbon.
Stability of cis-trans isomers:
As is illustrated in the above example of cis and trans-2-butene, always trans-2-butene is more stable than cis-2-butene. It is because in cis-2-butene there is a functional group on the same side of carbon that increases the steric hindrance between the two functional groups and so the electronic cloud is dispersed. Therefore, it is also noticed that in cis-2-butene there is less symmetry than in trans-2-butene. on the other hand, trans-2-butene is more stable than cis-2-butene because the functional groups are on the opposite side of the carbon which reduces the inter repulsion and causes stability.
CIS-ISOMERS <<<<< TRANS-ISOMERS
The density, refractive index, solubility, heat of combustion, dipole moment, and dissociation constant of cis-isomers are greater than that of trans-isomers.
| FEATURES | CIS-2-BUTENE | TRANS-2-BUTENE |
| 1. Density 2. Refractive index 3. Solubility 4. Melting point 5. Dipole moment | 0.641 g/ml (at 3.6 °C ) 1.47 658mg/l at 25 °C (Highly soluble) 4 °C 0.3 D | 0.626 g/ml (at 0.9 °C ) 1.384 at -25°C 1 mg/l at 25°C (slightly soluble) -138.9 °C No dipole moment |
E-Z Geometric isomerism:
The system of nomenclature of cis-trans geometrical isomers works very well for alkenes in which both carbons have a double bond and the same functional group either on the same side or on the different side at adjacent carbon. However, when there are three or four different groups attached to a carbon atom of the double bond it is difficult to assign a cis-trans designation to the geometrical isomers.
To remove this difficulty, a new system of nomenclature called E-Z isomerism is introduced which is based on the CIP (Cahn-Ingold-Prelog) scheme which is applied to chiral molecules and applies to all cases of geometric isomerism. In E-Z isomers, E-isomers correspond/equivalent to trans-form and Z-isomers correspond/equivalent to cis form.
CIP Scheme & E-Z isomers:
Cahn-Ingold-Prelog (CIP) scheme is the scheme that is used to give the names to E-Z isomers. According to the CIP schemes:
- The two groups on each carbon of double bond are ranked in order of priority as was done in the case of chiral molecules.
- This priority is assigned to each carbon of pie bond on basis of their atomic weight.
- If both groups of higher priority are on the same side of the double bond, the geometrical isomers are designated as the Z-isomers. “Z” comes from the German word Zusammen which means together.
- If both groups of higher priority are on the opposite side of the double bond, the geometrical isomers are designated as the E-isomers. “E” comes from the German word entgegen meaning opposite.
- If all the elements or atoms have the same atomic weight, then we will compare the atomic weight of the next atom.
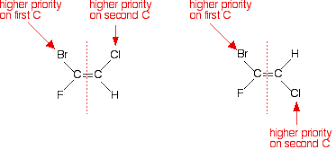
This diagram is showing the higher priority based on atomic weight between Cl & H or Br & F.
- E-Z isomerism is also shown in the minor addition to the isotopes. Hydrogen possesses three isotopes protium, deuterium, and tritium. Deuterium has more atomic weight than H. So, in this case, D is given high priority and on the other side, chlorine is given more priority.

These both are E-Z isomers that have D and Cl as a higher priority.
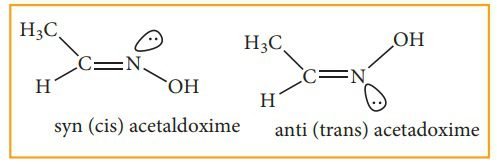
Syn-anti Isomerism:
Syn-anti isomerism is carried out by the molecules that possess carbon-nitrogen (C=N), and nitrogen-nitrogen (N=N) double bonds. Syn-anti isomerism is carried out in oximes and azo compounds. We shall discuss in detail the oxime and azo compounds. The basic condition for this syn-anti isomerism is restricted rotation. There is restricted rotation in the C=N or N=N double bonds.
OXIMES are the compounds that are formed by the reaction of carbonyl compound and hydroxylamine group. In this reaction, dehydration occurs so that water is removed and the remaining product obtained is oxime.

AZO COMPOUNDS are compounds that have a diezynyl group in them. In the diezynyl group, there are two R groups R and R’ these two groups are maybe the alkyl or aryl groups. A nitrogen-nitrogen (N=N) double bond exists between the azo compounds.
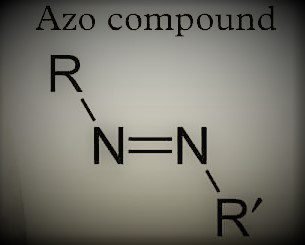
In this isomerism, “Syn” is used as a cis form and “Anti” is used as a trans-form. In this, we cannot differentiate syn-anti isomers by locating functional groups on the same or different sides. We can differentiate it with the help of -H and -OH functional groups. If -H and -OH are on different sides then the molecule is considered an Anti-isomer. Rather, if these both are on the same side they are syn-isomers. In nomenclature, there prefixes syn and anti are adapted instead of cis and trans. In this syn-oxime is introduced when hydroxyl group -OH on nitrogen and -H group on carbon is introduced on the same side and in anti-isomer these both groups are on the opposite side.
Interconversion of Geometrical isomers:
Although geometrical isomers are quite stable, the interconversions (cis-trans) about double bonds can be carried out by creating some degree of single bond characters in the double bonds. This can be accomplished by the excitation of molecules by heat, or by exposure to light, or transitory addition.
For example: when a solution of maleic acid in aqueous HCl is heated, the acid is isomerized to fumaric by the way of reversible protonation. The equilibrium that is established lies on the side of more stable trans-acids.



Leave a Reply